Abstract
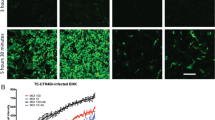
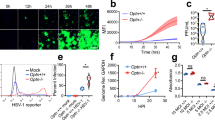
The Bax protein is widely known as a pro-apoptotic Bcl-2 family member that when overexpressed can trigger apoptosis in multiple cell types and is important for the developmental cell death of neurons1,2. However, Bax was found here to be a potent inhibitor of neuronal cell death in mice infected with Sindbis virus. Newborn mice, which are highly susceptible to a fatal infection with neurotropic Sindbis virus, were significantly protected from neuronal apoptosis and fatal disease when infected with a recombinant Sindbis virus encoding Bax. Deletion of the N terminus of Bax, which mimics cleaved Bax, converted Bax into a pro-apoptotic factor in vivo. As mice mature during the first week after birth, they acquire resistance to a fatal Sindbis virus infection3,4. However, Bax-deficient mice remained very sensitive to fatal disease compared with their control littermates, indicating that endogenous Bax functions as a survival factor and contributes to age-dependent resistance to Sindbis virus-induced mortality. The protective effects of Bax were reproduced in cultured hippocampal neurons but not in cultured dorsal root ganglia neurons. These findings indicate that cell-specific factors determine the anti-apoptotic versus pro-apoptotic function of Bax.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Deckwerth, T.L. et al. Bax is required for neuronal death after trophic factor deprivation and during development. Neuron 17, 401–411 (1996).
White, F.A., Keller-Peck, C.R., Knudson, C.M., Korsmeyer, S.J. & Snider, W.D. Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J. Neurosci. 18, 1428–1439 (1998).
Lewis, J., Wesselingh, S.L., Griffin, D.E. & Hardwick, J.M. Sindbis virus-induced apoptosis in mouse brains correlates with neurovirulence. J. Virol. 70, 1828–1835 (1996).
Hardwick, J.M., Irani, D.N. & Griffin, D.E. in Cell Death in Diseases of the Nervous System (eds. Koliatsos, V.E. & Ratan, R.) 295–323 (Humana Press, Totowa, New Jersey, 1999).
Middleton, G., Nunez, G. & Davies, A.M. Bax promotes neuronal survival and antagonises the survival effects of neurotrophic factors. Development 122, 695–701 (1996).
Oh, J.H., O'Malley, K.L., Krajewski, S., Reed, J.C. & Oh, Y.J. Bax accelerates staurosporine-induced but suppresses nigericin-induced neuronal cell death. Neuroreport 8, 1851–1856 (1998).
Zhou, M., Demo, S.D., McClure, T.N., Crea, R. & Bitler, C.M. A novel splice variant of the cell death-promoting protein BAX. J. Biol. Chem. 273, 11930–11936 (1998).
Cheng, E.H.-Y., Levine, B., Boise, L.H., Thompson, C.B. & Hardwick, J.M. Bax-independent inhibition of apoptosis by Bcl-xL. Nature 379, 554–556 (1996).
Levine, B., Goldman, J.E., Jiang, H.H., Griffin, D.E. & Hardwick, J.M. Bcl-2 protects mice against fatal alphavirus encephalitis. Proc. Natl. Acad. Sci. USA 93, 4810–4815 (1996).
Levine, B. et al. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature 361, 739–742 (1993).
Ubol, S., Tucker, P.C., Griffin, D.E. & Hardwick, J.M. Neurovirulent strains of alphavirus induce apoptosis in bcl-2-expressing cells; Role of a single amino acid change in the E2 glycoprotein. Proc. Natl. Acad. Sci. USA 91, 5202–5206 (1994).
Hunter, J.J., Bond, B.L. & Parslow, T.G. Functional dissection of the human Bcl-2 protein: sequence requirements for inhibition of apoptosis. Mol. Cell. Biol. 16, 877–883 (1996).
Cheng, E.H.-Y. et al. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science 278, 1966–1968 (1997).
Clem, R. J. et al. Modulation of cell death by Bcl-xL through caspase interaction. Proc. Natl. Acad. Sci. USA 95, 554–559 (1998).
Yoshida, H. et al. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell 94, 739–750 (1998).
Kuida, K. et al. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell 94, 325–337 (1998).
Chou, J.J., Li, H., Salvesen, G., Yuan, J. & Wagner, G. Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell 96, 615–624 (1999).
McDonnell, J.M., Fushman, D., Milliman, C.L., Korsmeyer, S.J. & Cowburn, D. Solution structure of the proapoptotic molecule BID: a structural basis for apoptotic agonists and antagonists. Cell 96, 625–634 (1999).
Thomas, A. et al. Drug-induced apoptosis in B-cell chronic lymphocytic leukemia: relationship between p53 gene mutation and bcl-2/bax proteins in drug resistance. Oncogene 12, 1055–1062 (1996).
Grandgirard, D. et al. Alphaviruses induce apoptosis in Bcl-2-overexpressing cells: evidence for a caspase-mediated, proteolytic inactivation of Bcl-2. EMBO J. 17, 1268–1278 (1998).
Wood, D.E. et al. Bax cleavage is mediated by calpain during drug-induced apoptosis. Oncogene 17, 1069–1078 (1998).
Kirsch, D.G. et al. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J. Biol. Chem. (in the press).
Wolter, K.G. et al. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell. Biol. 139, 1281–1292 (1997).
Kiefer, M.C. et al. Modulation of apoptosis by the widely distributed Bcl-2 homologue Bak. Nature 374, 736–739 (1995).
Vornov, J.J., Tasker, R.C. & Park, J. Neurotoxicity of acute glutamate transport blockade depends on coactivation of both NMDA and AMPA/Kainate receptors in organotypic hippocampal cultures. Exp. Neurol. 133, 7–17 (1995).
Acknowledgements
This work was supported by research grant NS34175 (J.M.H.) from the National Institutes of Health and a career development award from the Burroughs Wellcome Foundation (G.A.O.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lewis, J., Oyler, G., Ueno, K. et al. Inhibition of virus-induced neuronal apoptosis by Bax. Nat Med 5, 832–835 (1999). https://doi.org/10.1038/10556
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/10556
This article is cited by
-
In vivo CaspaseTracker biosensor system for detecting anastasis and non-apoptotic caspase activity
Scientific Reports (2015)
-
N-terminally cleaved Bcl-xL mediates ischemia-induced neuronal death
Nature Neuroscience (2012)
-
MicroRNA-128 downregulates Bax and induces apoptosis in human embryonic kidney cells
Cellular and Molecular Life Sciences (2011)
-
Apoptosis induced by Semliki Forest virus is RNA replication dependent and mediated via Bak
Cell Death & Differentiation (2008)
-
Mitochondrial localization and pro-apoptotic effects of the interferon-inducible protein ISG12a
Apoptosis (2008)