Abstract
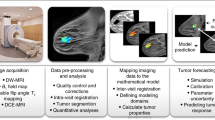
Magnetic resonance imaging (MRI) is a noninvasive method that reveals anatomical details in vivo and detects lesions for diagnosis. Although standard breast MRI cannot clearly delineate breast cancer, contrast-enhanced MRI enables the detection of breast masses with high sensitivity1,2. Dynamic studies demonstrated that malignant lesions were characterized by a faster signal enhancement rate than benign ones3. Dynamic MRI of human breast cancer in mice revealed high heterogeneity in the distribution of contrast-enhanced curves and derived pathophysiological features, indicating the importance of high spatial resolution4,5. With clinical MRI, it is difficult to achieve simultaneously high spatial and temporal resolution. In previous dynamic studies, the emphasis was on high temporal resolution and mainly empiric analyses6–12. We describe here a new model-based method that optimizes spatial resolution by using only three time points, and yet characterizes tumor heterogeneity in terms of microvascular permeability and extracellular fraction. Mapping these pathophysiological features may aid diagnosis and prognosis assessment, while the high spatial resolution may improve the capacity to detect smaller lesions. The method was tested in human breast tumors implanted in mice and in a limited number of benign and malignant breast lesions of patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Harms, S.E. & Flaming, D.P. MR imaging of the breast. J. Magn. Reson. Imaging 3, 277–283 (1993).
Heywang-Kobrunner, S.H. Contrast-enhanced magnetic resonance imaging of the breast. Invest. Radiol. 29, 94–104 (1994).
Kaiser, W.A. & Zeitler, E. MR imaging of the breast: Fast imaging sequences with and without Gd-DTPA. Radiology 170, 681–686 (1989).
Furman-Haran, E., Margalit, R., Maretzek, A.F. & Degani, H. Angiogenic response of MCF7 human breast cancer to hormonal treatment: Assessment by dynamic GdDTPA-enhanced MRI at high spatial resolution. J. Magn. Reson. Imaging 6, 195–202 (1996).
Furman-Haran, E., Margalit, R., Grobgeld, D. & Degani, H. Dynamic contrast enhanced magnetic resonance imaging reveals stress induced angiogenesis in MCF7 human breast tumors. Proc. Natl. Acad. Sci. USA 93, 6247–6251 (1996).
Frouge, C., Guinebretiere, J.-M., Contesso, G., DiPaola, R. & Blery, M. Correlation between contrast enhancement in dynamic magnetic resonance imaging of the breast and tumour angiogenesis. Invest Radiol. 29, 1043–1049 (1994).
Buckley, D.L., Kerslake, R.W., Blackband, S.J. & Horsman, A. Quantitative analysis of multislice Gd-DTPA enhanced dynamic MR images using an automated simplex minimization procedure. Magn. Reson. Med. 32, 646–65 (1994).
Hoffmann, U., Brix, G., Knopp, M.V., Heb, T. & Lorenz, W.J. Pharmacokinetic mapping of the breast: A new method for dynamic MR mammography. Magn. Reson. Med. 33, 506–514 (1995).
Hulka, C.A. et al. Benign and malignant breast lesions: Differentiation with echo-planar MR imaging. Radiology 197, 33–38 (1995).
Tofts, P.S., Berkowitz, B. & Schnall, M.D. Quantitative analysis of dynamic Gd-DTPA enhancement in breast tumours using a permeability model. Magn. Reson. Med. 33, 564–568 (1995).
Lucas-Qesada, F.A., Sinha, U. & Sinha, S. Segmentation strategies for breast tumors from dynamic MR images. J. Magn. Reson. Imaging 6, 753–763 (1996).
Keltz, F., Santyr, G.E., Cron, G.O. & Mongin, S.J. Application of a quantitative model to differentiate benign from malignant breast lesions detected by dynamic, gadolinium-enhanced MRI. J. Magn. Reson. Imaging 6, 743–752 (1996).
Larsson, H.B.W. et al. Quantification of blood-brain barrier defect by magnetic resonance imaging and gadolinium-DTPA in patients with multiple sclerosis and brain tumors. Magn. Reson. Med. 16, 117–131 (1990).
Brix, G. et al. Pharmacokinetic parameters in CNS Gd-DTPA enhanced MR imaging. J. Computer Assisted Tomogr. 15, 621–628 (1991).
Tofts, P.S. & Kermode, A.G. Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn. Reson. Med. 17, 357–367 (1991).
Furman, E., Margalit, R., Bendel, P., Horowitz, A. & Degani, H. In vivo studies by magnetic resonance imaging and spectroscopy of the response to tamoxifen of MCF7 human breast cancer implanted in nude mice. Cancer Commun. 3, 287–297 (1991).
Folkman, J. Tumor angiogenesis. Advances Cancer Res. 43, 175–203 (1985).
Weidner, N., Semple, J.P., Wilch, W.R. & Folkman, J. Tumor angiogenesis and metastasis: Correlation in invasive breast carcinoma. N. Engl. J. Med. 324, 1–8 (1991).
Weidner, N. et al. Tumour angiogenesis: A new significant and independent prognostic indicator in early-stage breast carcinoma. 84, 1875–1887 (1992).
Degani, H. et al Evaluation of breast cancer therapy with contrast enhanced MRI at high spatial resolution. J. Magn. Reson. Imaging 4, 116 (1994).
Weidmann, H.J., Laniado, M. & Mutzel, W. Pharmacokinetics of GdDTPA/dimeglumine after intravenous injection into healthy volunteers. Physiol. Chem. Phys. Med. NMR 16, 167–172 (1984).
Poon, C.S., Bronskill, M.J., Hemkelman, R.M. & Boyd, N.F. Quantitative magnetic resonance imaging parameters and their relationship to mammographic pattern. J. Natl. Cancer Inst. 84, 777–781 (1992).
Lauffer, R.B. Paramagnetic metal complexes as water proton relaxation agents for NMR imaging: Theory and design. Chem. Rev. 87, 901–927 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Degani, H., Gusis, V., Weinstein, D. et al. Mapping pathophysiological features of breast tumors by MRI at high spatial resolution. Nat Med 3, 780–782 (1997). https://doi.org/10.1038/nm0797-780
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0797-780
This article is cited by
-
Integrated radiomic framework for breast cancer and tumor biology using advanced machine learning and multiparametric MRI
npj Breast Cancer (2017)
-
Differential diagnosis of benign and malignant breast masses using diffusion-weighted magnetic resonance imaging
World Journal of Surgical Oncology (2015)
-
Translational theranostic methodology for diagnostic imaging and the concomitant treatment of malignant solid tumors
Neurovascular Imaging (2015)