Abstract
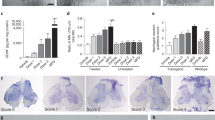
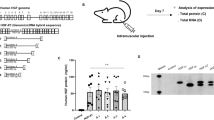
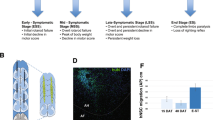
Neuronal growth factors hold promise for providing therapeutic benefits in various neurological disorders. As a means of ensuring adequate central nervous system delivery of growth factors and minimizing significant adverse side effects associated with systemic delivery methods, we have developed an ex vivo gene therapy approach for protein delivery using encapsulated genetically modified xenogeneic cells. Ciliary neurotrophic factor (CNTF) has been shown in various rodent models to reduce the motor neuron cell death similar to that seen in amyotrophic lateral sclerosis (ALS)1–3. The initial trials focusing on the systemic administration of CNTF for ALS have been discontinued as a result of major side effects, thus preventing determination of the potential efficacy of the molecule4,5. In order to deliver CNTF directly to the nervous system, we conducted a phase I study in which six ALS patients were implanted with polymer capsules containing genetically engineered baby hamster kidney cells releasing approximately 0.5 μg of human CNTF per day in vitro. The CNTF–releasing implants were surgically placed within the lumbar intrathecal space. Nanogram levels of CNTF were measured within the patients' cerebrospinal fluid (CSF) for at least 17 weeks post–transplantation, whereas it was undetectable before implantation. Intrathecal delivery of CNTF was not associated with the limiting side effects observed with systemic delivery. These results demonstrate that neurotrophic factors can be continuously delivered within the CSF of humans by an ex vivo gene therapy approach, opening new avenues for the treatment of neurological diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Mitsumoto, H. et al. Arrest of motor neuron disease in wobbler mice cotreated with CNTF and BDNF. Science 265, 1107–1110 (1994).
Sendtner, M. et al. Ciliary neurotrophic factor prevents degeneration of motor neurons in mouse mutant progressive motor neuronopathy. Nature 358, 502–504 (1992).
Sagot, Y. et al. Polymer encapsulated cell lines genetically engineered to release ciliary neurotrophic factor can slow down progressive motor neuropathy in the mouse. Eur. J. Neurosci. 7, 1313–1322 (1995).
Cederbaum, J.M. et al. The pharmacokinetics of subcutaneously administered recombinant human ciliary neurotrophic factor (rhCNTF) in patients with amyotrophic lateral sclerosis: Relationship to parameters of the acute phase response. Clin. Neuropharmacol. 18, 500–514 (1995).
Cederbaum, J.M. et al. A phase I study of recombinant human ciliary neurotrophic factor (rHCNTF) in patients with amyotrophic lateral sclerosis. Clin. Neuropharmacol. 18, 515–532 (1995).
Dittrich, F., Thoenen, H. & Sendtner, M. Ciliary neurotrophic factor: Pharmacokinetics and acute-phase response in rat. Ann. Neurol. 35, 151–163 (1994).
Aebischer, P. & Lysaght, M.J. Imunoisolation and cellular xenotransplantation. Xeno 3, 43–48 (1995).
Aebischer, P. et al. Gene therapy for amyotrophic lateral sclerosis (ALS) using a polymer encapsulated xenogenic cell line engineered to secrete hCNTF. Hum. Gene Ther. 7, 851–860 (1996).
Andres, P.L., Finison, L.J., Conlon, T., Thibobeau, L.M. & Munsat, T.L. Quantitative motor assessment in amyotrophic lateral sclerosis. Neurology 36, 937–941 (1986).
Norris, F.H., Calanchini, P.R., Fallat, R.J., Panchari, S. & Jewett, B. The administration of guanidine in amyotrophic lateral sclerosis. Neurology 24, 721–728 (1974).
Lewis, M.E. et al. Insulin-like growth factor-I: Potential for treatment of motor neuronal disorders. Exp. Neurol. 124, 73–88 (1993).
Hughes, R.A., Sendtner, M. & Thoenen, H. Members of several gene families influence survival of rat motoneurons in vitro and in vivo. J. Neurosci. Res. 36, 663–671 (1993).
Zurn, A.D., Baetge, E.E., Hammang, J.P., Tan, S.A. & Aebischer, P. Glial cell line-derived neurotrophic factor (GDNF), a new neurotrophic factor for motoneurones. NeuroReport 6, 113–118 (1994).
Henderson, C.E. et al. GDNF: A potent survival factor of motoneurons present in peripheral nerve and muscle. Science 266, 1062–1064 (1994).
Wong, V., Arriaga, R., Ip, N.Y. & Lindsay, R.M. The neurotrophins BDNF, NT-3 and NT-4/5, but not NGF, up-regulate the cholinergic phenotype of developing motor neurons. Eur. J. Neurosci. 5, 466–474 (1993).
Kato, A.C. & Lindsay, R.M. Overlapping and additive effects of neurotrophins and CNTF on cultured human spinal cord neurons. Exp. Neurol. 130, 196–201 (1994).
Sendtner, M., Holtmann, B., Kolbeck, R., Thoenen, H. & Barde, Y.A. Brain-derived neurotrophic factor prevents the death of motor neurons in newborn rats after nerve section. Nature 360, 757–759 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Aebischer, P., Schluep, M., Déglon, N. et al. Intrathecal delivery of CNTF using encapsulated genetically modifiedxenogeneic cells in amyotrophic lateral sclerosis patients. Nat Med 2, 696–699 (1996). https://doi.org/10.1038/nm0696-696
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0696-696
This article is cited by
-
Portable bioluminescent platform for in vivo monitoring of biological processes in non-transgenic animals
Nature Communications (2021)
-
3D Printed porous polyamide macrocapsule combined with alginate microcapsules for safer cell-based therapies
Scientific Reports (2018)
-
Effect of human very low-density lipoproteins on cardiotrophin-like cytokine factor 1 (CLCF1) activity
Scientific Reports (2018)
-
The Neuroprotective Agent CNTF Decreases Neuronal Metabolites in the Rat Striatum: An in Vivo Multimodal Magnetic Resonance Imaging Study
Journal of Cerebral Blood Flow & Metabolism (2015)
-
Preparation of calcium chloride-loaded solid lipid particles and heat-triggered calcium ion release
Korean Journal of Chemical Engineering (2015)