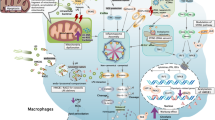
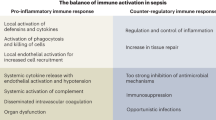
Abstract
The history of therapeutic interventions in clinical trials for sepsis has been referred to as the “graveyard for pharmaceutical companies.” That is now set to change, as research provides hope for new approaches that will be therapeutically effective in humans with sepsis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Angus, D.C. et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29, 1303–1310 (2001).
Bennett, I.L. et al. The effectiveness of hydrocortisone in the management of severe infection. JAMA 183, 462–465 (1963).
Lefering, R. & Neugebauer, E.A. Steroid controversy in sepsis and septic shock: a meta-analysis. Crit. Care Med. 23, 1294–1303 (1995).
Annane, D. et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 288, 862–871 (2002).
Meduri, G.U. et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA 280, 159–165 (1998).
Meduri, G.U., Tolley, E.A., Chrousos, G.P. & Stentz, F. Prolonged methylprednisolone treatment suppresses systemic inflammation in patients with unresolving acute respiratory distress syndrome: evidence for inadequate endogenous glucocorticoid secretion and inflammation-induced immune cell resistance to glucocorticoids. Am. J. Respir. Crit. Care Med. 165, 983–991 (2002).
Meduri, G.U., Kanangat, S., Stefan, J., Tolley, E. & Schaberg, D. Cytokines IL-1β, IL-6, and TNF-α enhance in vitro growth of bacteria. Am. J. Respir Crit. Care Med. 160, 961–967 (1999).
Kanangat, S. et al. Effects of cytokines and endotoxin on the intracellular growth of bacteria. Infect. Immun. 67, 2834–2840 (1999).
Davis, C.E., Brown, K.R., Douglas, H., Tate, W.J & Braude, A.I. Prevention of death from endotoxin with antisera. I. The risk of fatal anaphylaxis to endotoxin. J. Immunol. 102, 563–572 (1969).
Cohen, J. Adjunctive therapy in sepsis: a critical analysis of the clinical trial programme. Br. Med. Bull. 55, 212–225 (1999).
Bone, R.C. et al. A second large controlled clinical study of E5, a monoclonal antibody to endotoxin: results of a prospective, multicenter, randomized, controlled trial. The E5 Sepsis Study Group. Crit. Care Med. 23, 994–1006 (1995).
Ziegler, E.J. et al. Treatment of Gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N. Engl. J. Med. 324, 429–436 (1991).
Warren, H.S. et al. Assessment of ability of murine and human anti-lipid A monoclonal antibodies to bind and neutralize lipopolysaccharide. J. Exp. Med. 177, 89–97 (1993).
Michie, H.R. et al. Tumor necrosis factor and endotoxin induce similar metabolic responses in human beings. Surgery 104, 280–286 (1988).
Beutler, B., Milsark, I.W. & Cerami, A.C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science 229, 869–871 (1985).
Tracey, K.J. et al. Shock and tissue injury induced by recombinant human cachectin. Science 234, 470–474 (1986).
Tracey, K.J. et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330, 662–664 (1987).
Reinhart, K. & Karzai, W. Anti-tumor necrosis factor therapy in sepsis: update on clinical trials and lessons learned. Crit. Care Med. 29, S121–S125 (2001).
Okusawa, S., Gelfand, J.A., Ikejima, T., Connolly, R.J. & Dinarello, C.A. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J. Clin. Invest. 81, 1162–1172 (1988).
Ohlsson, K., Bjork, P., Bergenfeldt, M., Hageman, R. & Thompson, R.C. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature 348, 550–552 (1990).
Fisher, C.J., Jr. et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA 271, 1836–1843 (1994).
Opal, S.M. et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit. Care Med. 25, 1115–1124 (1997).
Dhainaut, J.F. et al. Platelet-activating factor receptor antagonist BN 52021 in the treatment of severe sepsis: a randomized, double-blind, placebo-controlled, multicenter clinical trial. BN 52021 Sepsis Study Group. Crit. Care Med. 22, 1720–1728 (1994).
Dhainaut, J.F. et al. Confirmatory platelet-activating factor receptor antagonist trial in patients with severe Gram-negative bacterial sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. BN 52021 Sepsis Investigator Group. Crit. Care Med. 26, 1963–1971 (1998).
Bernard, G.R. et al. The effects of ibuprofen on the physiology and survival of patients with sepsis. The Ibuprofen in Sepsis Study Group. N. Engl. J. Med. 336, 912–918 (1997).
Vincent, J.L. et al. A multi-centre, double-blind, placebo-controlled study of liposomal prostaglandin E1 (TLC C-53) in patients with acute respiratory distress syndrome. Intensive Care Med. 27, 1578–1583 (2001).
Yu, M. & Tomasa, G. A double-blind, prospective, randomized trial of ketoconazole, a thromboxane synthetase inhibitor, in the prophylaxis of the adult respiratory distress syndrome. Crit. Care Med. 21, 1635–1642 (1993).
Spies, C.D. et al. Influence of N-acetylcysteine on indirect indicators of tissue oxygenation in septic shock patients: results from a prospective, randomized, double-blind study. Crit. Care Med. 22, 1738–1746 (1994).
Schilling, J., Cakmakci, M., Battig, U. & Geroulanos, S. A new approach in the treatment of hypotension in human septic shock by NG-monomethyl-l-arginine, an inhibitor of the nitric oxide synthetase. Intensive Care Med. 19, 227–231 (1993).
Lorente, J.A., Landin, L., De Pablo, R., Renes, E. & Liste, D. L-arginine pathway in the sepsis syndrome. Crit. Care Med. 21, 1287–1295 (1993).
Avontuur, J.A., Tutein Nolthenius, R.P., van Bodegom, J.W. & Bruining, H.A. Prolonged inhibition of nitric oxide synthesis in severe septic shock: a clinical study. Crit. Care Med. 26, 660–667 (1998).
Fein, A.M. et al. Treatment of severe systemic inflammatory response syndrome and sepsis with a novel bradykinin antagonist, deltibant (CP-0127). Results of a randomized, double-blind, placebo-controlled trial. CP-0127 SIRS and Sepsis Study Group. JAMA 277, 482–487 (1997).
Staubach, K.H. et al. Effect of pentoxifylline in severe sepsis: results of a randomized, double-blind, placebo-controlled study. Arch. Surg. 133, 94–100 (1998).
Zeni, F. et al. Effects of pentoxifylline on circulating cytokine concentrations and hemodynamics in patients with septic shock: results from a double-blind, randomized, placebo-controlled study. Crit. Care Med. 24, 207–214 (1996).
Fronhoffs, S. et al. The effect of C1-esterase inhibitor in definite and suspected streptococcal toxic shock syndrome. Report of seven patients. Intensive Care Med. 26, 1566–1570 (2000).
Healy, D.P. New and emerging therapies for sepsis. Ann. Pharmacother. 36, 648–654 (2002).
van der Poll, T. Immunotherapy of sepsis. Lancet Infect. Dis. 1, 165–174 (2001).
Vincent, J.L., Sun, Q. & Dubois, M.J. Clinical trials of immunomodulatory therapies in severe sepsis and septic shock. Clin. Infect. Dis. 34, 1084–1093 (2002).
Warren, B.L. et al. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA 286, 1869–1878 (2001).
Bernard, G.R. et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344, 699–709 (2001).
Warren, H.S., Suffredini, A.F., Eichacker, P.Q. & Munford, R.S. Risks and benefits of activated protein C treatment for severe sepsis. N. Engl. J. Med. 347, 1027–1030 (2002).
Riewald, M., Petrovan, R.J., Donner, A., Mueller, B.M. & Ruf, W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science 296, 1880–1882 (2002).
Bustin, M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol. Cell. Biol. 19, 5237–5246 (1999).
Wang, H., Yang, H., Czura, C.J., Sama, A.E. & Tracey, K.J. HMGB1 as a late mediator of lethal systemic inflammation. Am. J. Respir. Crit. Care Med. 164, 1768–1773 (2001).
Andersson, U. et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 192, 565–570 (2000).
Wang, H. et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251 (1999).
Fiuza, C. et al. Inflammatory promoting activity of HMGB1 on human microvascular endothelial cells. Blood 101, 2652–2660(2002).
Sappington, P.L. et al. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology 123, 790–802 (2002).
Ulloa, L. et al. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc. Natl. Acad. Sci. USA 99, 12351–12356 (2002).
Calandra, T., Bernhagen, J., Mitchell, R.A. & Bucala, R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J. Exp. Med. 179, 1895–1902 (1994).
Bernhagen, J. et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature 365, 756–759 (1993).
Bozza, M. et al. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J. Exp. Med. 189, 341–346 (1999).
Calandra, T. et al. MIF as a glucocorticoid-induced modulator of cytokine production. Nature 377, 68–71 (1995).
Calandra, T. et al. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat. Med. 6, 164–170 (2000).
Gando, S. et al. Macrophage migration inhibitory factor is a critical mediator of systemic inflammatory response syndrome. Intensive Care Med. 27, 1187–1193 (2001).
Lehmann, L.E. et al. Plasma levels of macrophage migration inhibitory factor are elevated in patients with severe sepsis. Intensive Care Med. 27, 1412–1415 (2001).
Satoskar, A.R., Bozza, M., Rodriguez Sosa, M., Lin, G. & David, J.R. Migration-inhibitory factor gene-deficient mice are susceptible to cutaneous Leishmania major infection. Infect. Immun. 69, 906–911 (2001).
Koebernick, H. et al. Macrophage migration inhibitory factor (MIF) plays a pivotal role in immunity against Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 99, 13681–13686 (2002).
Calandra, T., Spiegel, L.A., Metz, C.N. & Bucala, R. Macrophage migration inhibitory factor is a critical mediator of the activation of immune cells by exotoxins of Gram-positive bacteria. Proc. Natl. Acad. Sci. USA 95, 11383–11388 (1998).
Mitchell, R.A. et al. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc. Natl. Acad. Sci. USA 99, 345–350 (2002).
Roger, T., David, J., Glauser, M.P. & Calandra, T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature 414, 920–924 (2001).
Shin, H.S., Snyderman, R., Friedman, E., Mellors, A. & Mayer, M.M. Chemotactic and anaphylatoxic fragment cleaved from the fifth component of guinea pig complement. Science 162, 361–363 (1968).
Goldstein, I.M. & Weissmann, G. Generation of C5-derived lysosomal enzyme-releasing activity (C5a) by lysates of leukocyte lysosomes. J. Immunol. 113, 1583–1588 (1974).
Sacks, T., Moldow, C.F., Craddock, P.R., Bowers, T.K. & Jacob, H.S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J. Clin. Invest. 61, 1161–1167 (1978).
Schumacher, W.A., Fantone, J.C., Kunkel, S.E., Webb, R.C. & Lucchesi, B.R. The anaphylatoxins C3a and C5a are vasodilators in the canine coronary vasculature in vitro and in vivo. Agents Actions 34, 345–349 (1991).
Guo, R.F. et al. Protective effects of anti-C5a in sepsis-induced thymocyte apoptosis. J. Clin. Invest. 106, 1271–1280 (2000).
Riedemann, N.C. et al. C5a receptor and thymocyte apoptosis in sepsis. FASEB J. 16, 887–888 (2002).
Huber-Lang, M. et al. Role of C5a in multiorgan failure during sepsis. J. Immunol. 166, 1193–1199 (2001).
Czermak, B.J. et al. Protective effects of C5a blockade in sepsis. Nat. Med. 5, 788–792 (1999).
Riedemann, N.C. et al. Increased C5a receptor expression in sepsis. J. Clin. Invest. 110, 101–108 (2002).
Huber-Lang, M.S. et al. Complement-induced impairment of innate immunity during sepsis. J. Immunol. 169, 3223–3231 (2002).
Gerard, N.P. & Gerard, C. The chemotactic receptor for human C5a anaphylatoxin. Nature 349, 614–617 (1991).
Riedemann, N.C. et al. Protective effects of IL-6 blockade in sepsis are linked to reduced C5a receptor expression. J. Immunol. 170, 503–507 (2003).
Fitch, J.C. et al. Pharmacology and biological efficacy of a recombinant, humanized, single-chain antibody C5 complement inhibitor in patients undergoing coronary artery bypass graft surgery with cardiopulmonary bypass. Circulation 100, 2499–2506 (1999).
Oberholzer, C., Oberholzer, A., Clare-Salzler, M. & Moldawer, L.L. Apoptosis in sepsis: a new target for therapeutic exploration. FASEB J. 15, 879–892 (2001).
Hotchkiss, R.S. et al. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc. Natl. Acad. Sci. USA 96, 14541–14546 (1999).
Hotchkiss, R.S. et al. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J. Immunol. 162, 4148–4156 (1999).
Hotchkiss, R.S. et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J. Immunol. 166, 6952–6963 (2001).
Eichacker, P.Q. et al. Risk and the efficacy of antiinflammatory agents: retrospective and confirmatory studies of sepsis. Am. J. Respir. Crit. Care Med. 166, 1197–1205 (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riedemann, N., Guo, RF. & Ward, P. Novel strategies for the treatment of sepsis. Nat Med 9, 517–524 (2003). https://doi.org/10.1038/nm0503-517
Issue Date:
DOI: https://doi.org/10.1038/nm0503-517
This article is cited by
-
N6-methyladenosine of Spi2a attenuates inflammation and sepsis-associated myocardial dysfunction in mice
Nature Communications (2023)
-
Argon inhalation attenuates systemic inflammation and rescues lung architecture during experimental neonatal sepsis
Pediatric Surgery International (2023)
-
During Sepsis and COVID-19, the Pro-Inflammatory and Anti-Inflammatory Responses Are Concomitant
Clinical Reviews in Allergy & Immunology (2023)
-
In silico molecular docking validation of procalcitonin-binding aptamer and sepsis diagnosis
Molecular & Cellular Toxicology (2023)
-
Commentary on Posterior Reversible Encephalopathy Syndrome and Sepsis-Associated Encephalopathy
Neurocritical Care (2022)