Abstract
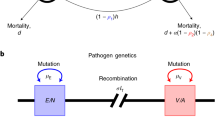
Using mathematical models that combine population genetic and epidemiological processes, we resolve the paradox that many important pathogens appear to persist as discrete strains despite the constant exchange of genetic material. We show that dominant polymorphic determinants (that is, those that elicit the most effective immune responses) will be organized into nonoverlapping combinations as a result of selection by the host immune system, thereby defining a set of discrete independently transmitted strains. By analysing 222 isolates of Neisseria meningitidis, we show that two highly polymorphic epitopes of the outer membrane protein PorA exist in nonoverlapping combinations as predicted by this general framework. The model indicates that dominant polymorphic determinants will be in linkage disequilibrium, despite frequent genetic exchange, even though they may be encoded by several unlinked genes. This suggests that the detection of nonrandom associations between epitope regions can be employed as a novel strategem for identifying dominant polymorphic antigens.
Similar content being viewed by others
Article PDF
References
Gupta, S., Trenholme, K., Anderson, R.M. & Day, K.P. Antigenic diversity and the transmission dynamics of Plasmodium faldparum. Science 263, 961–963 (1994).
Louwagie, J. et al. Phylogenetic analysis of gag genes from 70 international HIV-1 isolates provides evidence for multiple genotypes. AIDS 7, 769–780 (1993).
Maynard Smith, J., Dowson, C.G. & Spratt, B.C. Localized sex in bacteria. Nature 349, 29–31 (1991)
Walliker, D. Genetic recombination in malaria parasites. Exp. Parasitol. 69, 303–309 (1989).
Robertson, D.L., Sharp, P.M., McCutchan, F.E. & Hahn, B.H. Recombination in HIV-1. Nature 374, 124–126 (1995).
Feavers, I.M., Heath, A.B., Bygraves, J.A. & Maiden, M.C.J. Role of horizontal genetic exchange in the antigenic variation of the class 1 outer membrane protein of Neisseria meningitidis. Mol Microbiol. 6, 489–495 (1992)
Desjardins, P., Picard, B., Kaltenbock, B., Elion, J. & Denamur, E. Sex in Escherichia coli does not disrupt the clonal structure of the population: Evidence from random amplified polymorphic DNA and restriction-fragment-length polymorphism. J. Mol Evol. 41, 440–448 (1995).
Levin, B. Periodic selection, infectious gene exchange and the genetic structure of E. coli populations. Genetics 99, 1–23 (1981).
Hartl, D. & Clark, A.G. Principles of Population Genetics (Sinauer Associates, Sunderland, Massachusetts, 1989).
Anderson, R.M. & May, R.M. Infectious Diseases of Humans: Dynamics & Control (Oxford Univ. Press, Oxford, 1991).
Feavers, I.M. et al. Antigenic diversity of meningococcal outer membrane protein PorA has implications for epidemiological analysis and vaccine design Clin. Diagn. Lab. Immunol (in the press).
Suker, J. et al. The porA gene in serogroup A meningococci: Evolutionary stability and mechanism of genetic variation. Mol. Microbiol 12, 253–265 (1994).
Maiden, M.C.J. Population genetics of a transformable bacterium: The influence of horizontal genetic exchange on the biology of Neisseria meningitidis. FEMS Microbiol.Lett. 112, 243–250 (1993).
Maiden, M.C.J. & Feavers, I.M. Population genetics and global epidemiology of the human pathogen Neisseria meningitidis. in Population Genetics of Bacteria (eds. Baumberg, S., Young, J.P.W., Saunders, J.R. & Wellington, E.M.H.) 269–293 (Cambridge Univ. Press, Cambridge, 1995).
Maynard Smith, J.M., Smith, N.H., O'Rourke, M. & Spratt, B.G. How clonal are bacteria? Proc. Natl. Acad. Scl USA 90, 4384–4388 (1993).
Spratt, B.G., Smith, N.H., Zhou, J., O'Rourke, M. & Feil, E. The population genetics of the pathogenic Neisseria. in Population Genetics of Bacteria (eds. Baumberg, S., Young, J.P.W., Saunders, J.R. & Wellington, E.M.H.) 143 (Cambridge Univ. Press, Cambridge, 1995).
Cartwright, K.A.V., Stuart, J.M., Jones, D.M. & Noah, N.D. The Stonehouse survey: Nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol Infect 99, 591–601 (1987).
Craven, D.E. et al. Adherence of isolates of Neisseria meningitidis from patients and carriers to human buccal epithelial cells. J. Infect Dis. 142, 556–568 (1980).
Maiden, M.C.J., Suker, J., McKenna, A.J., Bygraves, J.A. & Feavers, I.M. Comparison of the class 1 outer membrane proteins of eight serological reference strains of Neisseria meningitidis. Mol. Microbiol. 5, 727–736 (1991).
Mandrell, R.E. & Zollinger, W.D. Human immune response to meningococcal outer membrane protein epitopes after natural infection or vaccination. Infect Immun. 57, 1590–1598 (1989).
Wiertz, E.J. et al. T cell recognition of Neisseria meningitidis class 1 outer membrane proteins. Identification of T cell epitopes with selected synthetic peptides and determination of HLA restriction elements. J. Immunol. 147, 2012–2018 (1991).
Hughes, A.L. Positive selection and interallelic recombination at the merozoite surface antigen-1 (MSA-1) locus of Plasmodium faldparum. Mol. Biol. Evol. 9, 381–393 (1992).
Gupta, S. & Day, K.P. A strain theory of malarial transmission. Parasitol. Today 10, 476–481 (1994).
Roberts, D.J. et al. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature 357, 689–692 (1992).
Tibayrenc, M. Antigenic diversity and the transmission dynamics of Plasmodium falciparum: The clonality/sexuality debate revisited. Parasitol Today 10, 456–457 (1994).
Baruch, D.I. et al. Cloning the P. faldparum gene encoding PfEMPl, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82, 77–87 (1995).
Su, X-z. et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium faldparum-intected erythrocytes. Cell 82, 89–100 (1995).
Smith, J.D. et al. Switches in expression of Plasmodium faldparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell 82, 101–110 (1995).
Hayes, L.J. et al. Extent and kinetics of genetic change in the ompl gene of Chlamydia trachomatis in two villages with endemic trachoma. J. Infect Dis. 172, 268–272 (1995).
Gupta, S. & Day, K.P. A theoretical framework for the immunoepidemiology of Plasmodium faldparum malaria. Parasite Immunol 16, 361–370 (1994).
Daly, T.M. & Long, C.A. A recombinant 15-kilodalton carboxyl-terminal fragment of Plasmodium yoelii yoelii 17XL merozoite surface protein 1 induces a protective immune response in mice. Infect. Immun. 61, 2462–2467 (1993).
Hoffman, S. et al. Naturally acquired antibodies to sporozoites do not prevent malaria: Vaccine development implications. Science 237, 639–642 (1987).
Jones, T.R., Ballou, W.R. & Hoffman, S.L. Antibodies to the circumsporozoite protein and protective immunity to malaria sporozoites. Prog. Clin. Parasitol. 3, 103–117 (1993).
Hill, A.V.S. et al. Molecular analysis of the association of HLA-B53 and resistance to severe malaria. Nature 360, 434–439 (1992).
Gupta, S., Swinton, J. & Anderson, R.M. Theoretical studies of the effects of genetic heterogeneity in the parasite population on the transmission dynamics of malaria. Proc. R. Soc. Series B 256, 231–238 (1994).
Gotschlich, E.C., Goldschneider, I. & Artenstein, M.S. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J. Exp. Med. 129, 1367–1384 (1969).
Wyle, F.A. et al. Immunologic response of man to group B meningococcal polysac-charide vaccines. J. Infect. Dis. 126, 514–521 (1972).
Carman, W.F., Thomas, H.C., Zuckerman, A.J. & Harrison, T. Hepatitis B virus: Molecular variants. in Viral Hepatitis (eds. Zuckerman, A.J. & Thomas, H.C.) 115–136 (Churchill Livingstone, Edinburgh, 1993)
Norder, H. et al. Genetic relatedness of hepatitis B viral strains of diverse geographical origin and natural variations in the primary structure of the surface antigen. J. Gen. Virol. 74, 1341–1348 (1993).
Bollyky, P.L., Rambaut, A., Harvey, P.H. & Holmes, E.C. Recombination between sequences of Hepatitis B Virus from different genotypes. J. Mol Evol (in the press).
Tibayrenc, M., Kjellberg, F. & Ayala, F.J. A clonal theory of parasitic protozoa: The population structures of Entamoeba, Giardia, Leishmania, Naegleriaf Plasmodium, Trichomonas, and Trypanosoma and their medical and taxonomical consequences Proc. Natl Acad. Scl USA 87, 2414–2418 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gupta, S., Maiden, M., Feavers, I. et al. The maintenance of strain structure in populations of recombining infectious agents. Nat Med 2, 437–442 (1996). https://doi.org/10.1038/nm0496-437
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0496-437
This article is cited by
-
Genomic and panproteomic analysis of the development of infant immune responses to antigenically-diverse pneumococci
Nature Communications (2024)
-
Maximum antigen diversification in a lyme bacterial population and evolutionary strategies to overcome pathogen diversity
The ISME Journal (2022)
-
Negative frequency-dependent selection and asymmetrical transformation stabilise multi-strain bacterial population structures
The ISME Journal (2021)
-
Antigenic escape selects for the evolution of higher pathogen transmission and virulence
Nature Ecology & Evolution (2021)
-
A Mathematical Modeling Study: Assessing Impact of Mismatch Between Influenza Vaccine Strains and Circulating Strains in Hajj
Bulletin of Mathematical Biology (2021)