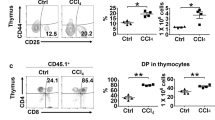
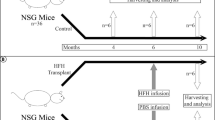
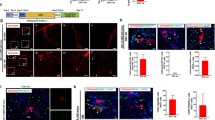
Abstract
Recently, cases have been reported in which a mixed chimeric state of blood cells is established after liver transplantation. Because the established chimerism may have aided in the induction of donor–specific tolerance, the mechanism responsible for this chimerism is of clinical importance. To establish this, we examined cells in adult mouse liver and identified the presence of c–kit+ Sca–1+ Linlo/− cells. These cells were capable of forming in vivo as well as in vitro colonies. Furthermore, the cells could reconstitute bone marrow of lethally irradiated recipient mice for at least 12 months. These data obtained from the mouse study strongly suggest that hematopoietic stem cells residing in the donor liver are responsible for mixed chimerism and maintenance of tolerance after liver transplantation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Billingham, R.E., Brent, L. & Medawar, P.B. Actively acquired tolerance of foreign cells. Nature 172, 603–605 (1953).
Starzl, T.E. et al. Cell migration, chimerism, and graft acceptance. Lancet 339, 1579–1582 (1992).
Starzl, T.E. et al. Chimerism after liver transplantation for type IV glycogen storage disease and type I Gauchers disease. N. Engl. J. Med. 328, 745–749 (1993).
Starzl, T.E. et al. Donor cell chimerism permitted by immimosuppressive drugs: A new view of organ transplantation. Immunol. Today. 14, 326–332 (1993).
Demetris, A.J. et al. Hematolymphoid cell trafficking, microchimerism, and GVHD reactions after liver, bone marrow, and heart transplantation. Transplant. Proc. 25, 3337–3344 (1993).
Qian, S. et al. Murine liver allograft transplantation: Tolerance and donor cell chimerism. Hepatology 19, 916–924 (1994).
Rasmussen, A. et al. Combined transplantation of liver and kidney from the same donor protects the kidney from rejection and improves kidney graft survival. 6th Congress of the European Society for Organ Transplantation, Rodos, Greece, Oct 25–28, 1993 (abstr.).
Starzl, T.E. et al. Evolution of liver transplantation. Hepatology 2, 614–624 (1982).
Markus, B.H. et al. Histocompatibility and liver transplnt outcome-Dose HLA exert a dualistic effect? Transplantation 46, 372–377 (1988).
Kamada, N., Bros, G. & Davies, H.S. Fully allogeneic liver grafting in rats induces a state of systemic nonreactivity to donor transplantation antigens. Transplantation 29, 429–431 (1980).
Kamada, N., Davies, H.S. & Koser, B. Reversal of transplantation immunity by liver grafting. Nature 292, 840–842 (1981).
Calne, R. & Davies, H.S. Organ graft tolerance: Liver effect. Lancet 343, 67–68 (1994).
Taniguchi, H., Toyoshima, T., Fukao, K. & Nakauchi, H. Evidence for the presence of hematopoietic stem cells in the adult liver. Transplant. Proc. 27, 196–199 (1995).
Okada, S. et al. In vivo and in vitro stem cell function of c-kit and Sca-1-positive murine hepopoietic cells. Blood 80, 3044–3050 (1992).
Ema, H., Suda, T., Miura, Y. & Nakauchi, H. Colony formation of clone-sorted human hematopoietic progenitors. Blood 75, 1941–1946 (1990).
Korbling, M. et al. Albumin density gradient purification of canine hemopoietic blood stem cells (HBSC): Long-term allogeneic engraftment without GVH-reaction. Exp. Hematol. 7, 277–288 (1979).
Schmitz, N. et al. Primary transplantation of allogeneic peripheral blood prog-enitor cells mobilized by filgrastim (granulocyte colony-stimulating factor). Blood. 85, 1666–1672 (1995).
Dunbar, C.E. et al. Retrovirally marked CD34-enriched peripheral blood and bone marrow cells contribute to long-term engraftment after autologous transplantation. Blood. 85, 3048–3057 (1995).
Brecher, G., Ansel, J.D., Micklem, H.S., Tjio, J.H. & Cronkite, E.P. Special proliferative sites are not needed for seeding and proliferation of transfused bone marrow cells in normal syngeneic mice. Proc. Natl. Acad. Sci. USA 79, 5085–5087 (1982).
Saxe, D.F., Boggs, S.S. & Boggs, D.R. Transplantation of chromosomally marked syngeneic marrow cells into mice not subjected to hematopoietic stem cell depletion. Exp. Hematol. 12, 277–283 (1984).
Stewart, F.M., Crittenden, R.B., Lowry, P.A., Pearson-White, S. & Quesenberry, P.J. Long-term engraftment of normal and post-5-fluorouracil murine marrow into normal nonmyeloablated mice. Blood 81, 2566–2571 (1993).
Lu, L., Xial, M., Shen, R., Grigsby, S. & Broxmeyer, H.E., Enrichment, characterization, and responsiveness of single primitive CD34+++ human umbilical cord blood hematopoietic progenitors with high proliferative and replating potential. Blood 81, 41–48 (1993).
Taniguchi, H., Jinzenji, Y., Fukao, K. & Nakauchi, H. Hematopoietic stem cell chimerism: Relationships between degree of chimerism, T cell clonal deletion, and graft survival. Transplant. Proc. 26, 1966–1968 (1994).
Taniguchi, H., Abe, M., Shirai, T., Fukao, K. & Nakauchi, H., Ratio is critical for alloreactive T cell deletion and skin graft survival in mixed bone marrow chimeras. J. Immunol. 155, 5631–5636 (1995).
Scheid, M.P. & Triglia, D. Further descripton of the Ly-5 system. Immunogenetics 9, 423–433 (1979).
Morce, H.C., Shen, F.W. & Hammerling, U. Nomenclature for loci controlling mouse lymphocyte antigens. Immunogenetics 23, 71–78 (1987).
Coffman, B. Surface antigen expression and immunoglobulin rearrangement during mouse pre-B cell development. Immunol. Rev. 69, 5–23 (1982).
Springer, T., Galfre, G., Secher, D.S. & Milstein, C. Mac-1: A macrophage differentiation antigen identified by monoclonal antibody. Eur. J. Immunol. 9, 301–306 (1979).
Holms, K.L. et al. Analysis of neoplasms induced by Cas-Br-M MuLV tumor extracts. J. Immunol. 137, 679–688 (1986).
Dialynas, D.P. et al. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1. 5: Expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol. Rev. 74, 29–56 (1983).
Ledbetter, J., Rouse, R., Micklem, H.S. & Herzenberg, L.A. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. J. Exp. Med. 152, 280–295 (1980).
Ikuta, K. et al. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell 62, 863–874 (1990).
Nishikawa, S. et al. In utero manipulation of coat color formation by a monoclonal anti-c-kit antibody: Two distinct waves of c-kit dependency during melanocyte development. EMBO J. 10, 2111–2118 (1991).
Spangrude, G.J., Heimfeld, S. & Weissman, I.L. Purification and characterization of mouse hematopoietic stem cells. Science 241, 58–62 (1988).
Shen, F.W. Monoclonal Antibodies and T-cell Hybridomas: Perspectives and Technical Advancesl (Amsterdam) 25 (Elsevier/North-Holland Biomedica, 1981).
Hardy, R.R. Purification and coupling of fluorescent proteins for use in flow cytometry. in Handbook of Experimental Immunology 4th edn (ed. Weir, D.M.) ch. 31 (Blackwell Scientific Publications, Oxford, 1986).
Philip, A.L., Zsebo, K.M., Deacon, D.H., Eichman, C.E. & Quesenberry, P.J. Effects of rrSCF on multiple cytokine responsive HPP-CFC generated from SCA+Lin- murine hematopoietic progenitors. Exp. Hematol. 19, 994–996 (1991).
Philip, A.L., Deacon, D.H., Whitefield, P., McGrath, H.E. & Quesenberry, P.J. Stem cell factor induction of in vitro murine hematopoietic colony formation by “subliminal” cytokine combinations: The role of “anchor factors“. Blood 80, 663–669 (1992).
Till, J.E. & McCulloch, E.A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat. Res. 14, 213–220 (1961).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Taniguchi, H., Toyoshima, T., Fukao, K. et al. Presence of hematopoietic stem cells in the adult liver. Nat Med 2, 198–203 (1996). https://doi.org/10.1038/nm0296-198
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0296-198
This article is cited by
-
Endothelial Jak3 expression enhances pro-hematopoietic angiocrine function in mice
Communications Biology (2021)
-
Inflammatory processes in the liver: divergent roles in homeostasis and pathology
Cellular & Molecular Immunology (2021)
-
Interferon gamma inhibits the differentiation of mouse adult liver and bone marrow hematopoietic stem cells by inhibiting the activation of notch signaling
Stem Cell Research & Therapy (2019)
-
Innate lymphoid cell memory
Cellular & Molecular Immunology (2019)
-
Basic liver immunology
Cellular & Molecular Immunology (2016)