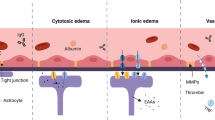
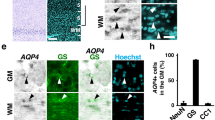
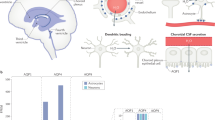
Abstract
Cerebral edema contributes significantly to morbidity and death associated with many common neurological disorders. However, current treatment options are limited to hyperosmolar agents and surgical decompression, therapies introduced more than 70 years ago. Here we show that mice deficient in aquaporin-4 (AQP4), a glial membrane water channel, have much better survival than wild-type mice in a model of brain edema caused by acute water intoxication. Brain tissue water content and swelling of pericapillary astrocytic foot processes in AQP4-deficient mice were significantly reduced. In another model of brain edema, focal ischemic stroke produced by middle cerebral artery occlusion, AQP4-deficient mice had improved neurological outcome. Cerebral edema, as measured by percentage of hemispheric enlargement at 24 h, was decreased by 35% in AQP4-deficient mice. These results implicate a key role for AQP4 in modulating brain water transport, and suggest that AQP4 inhibition may provide a new therapeutic option for reducing brain edema in a wide variety of cerebral disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Klatzo, I. Neuropathological aspects of brain edema. Acta Neurochir.Suppl. 60, 3–6 (1994).
Fishman, R. Brain edema. N. Eng. J. Med. 293, 706–711 (1975).
Weed, L.H. & McKibben, P.S. Experimental alteration of brain bulk. Am. J. Physiol. 48, 531–558 (1919).
Verkman, A.S. et al. Water transport across mammalian cell membranes. Am. J. Physiol. 48, C12–C30 (1996).
King, L.S. & Agre, P. Pathophysiology of the aquaporin water channels. Ann. Rev. Physiol. 58, 619–648 (1996).
Nielsen, S., Smith, B.L., Christensen, E.I. & Agre, P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc. Natl. Acad. Sci. USA 90, 7275–7279 (1993).
Hasegawa, H., Zhang, R., Dohrman, A. & Verkman, A.S. Tissue-specific expression of mRNA encoding the rat kidney water channel CHIP28k by in situ hybridization. Am. J. Physiol. 264, C237–C245 (1993).
Frigeri, A., Gropper, M., Turck, C.W. & Verkman, A.S. Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proc. Natl. Acad. Sci. USA 92, 4328–4331 (1995).
Nielsen, S. et al. Specialized membrane domains for water transport in glial cells: high- resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. Neuro. 17, 171–180 (1997).
Ma, T. et al. Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J. Clin. Invest. 100, 957–962 (1997).
Trachtman, H. Cell volume regulation: a review of cerebral adaptive mechanisms and implications for clinical treatment of osmolal disturbances. Ped. Nephrol. 1992, 104–112 (1992).
Hossman, K.A. Experimental models for the investigation of brain ischemia. Cardiovasc. Res. 39, 106–120 (1998).
Kondo, T. et al. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J. Neurosci. 17, 4180–4189 (1997).
Gullans, S.R. & Verbalis, J.G. Control of brain volume during hypoosmolar and hypoosmolar conditions. Annu. Rev, Med. 44, 289–301 (1993).
Wasterlain, C.G. & Torack, R.M. Cerebral edema in water intoxication. II. An ultrastructural study. Arch. Neurol. 19, 79–87 (1968).
Marmarou, A., Poll, W., Shulman, K. & Bhagavan, H. A simple gravimetric technique for measurement of cerebral edema. J. Neurosurg. 49, 530–537 (1978).
Nelson, S.R., Mantz, M.L. & Maxwell, J.A. Use of specific gravity in the measurement of cerebral edema. J. Appl. Physiol. 30, 268–271 (1971).
Hasegawa, H., Ma, T., Skach, W., Matthay, M. & Verkman, A.S. Molecular cloning of a mercurial-insensitive water channel expressed in selected water transporting tissues. J. Biol. Chem. 269, 5497–5500 (1994).
Jung, J.S. et al. Molecular characterization of an aquaporin cDNA from brain: candidate osmoreceptor and regulator of water balance. Proc. Natl. Acad. Sci. USA 91, 13052–13056 (1994).
Kimelberg, H. K. Current concepts of brain edema. Review of laboratory investigations. J. Neuro. 83, 1051–1059 (1995).
Bullock, R., Maxwell, W.L. & Graham, D.I. Glial swelling following cerebral contusion: an ultrstructural study. J. Neurol. Neurosurg. Psychiatry 54, 427–434 (1991).
Wasterlain, C.G. & Posner, J.B. Cerebral edema in water intoxication. I. Clinical and chemical observations. Arch. Neurol. 19, 71–78 (1968).
Landis, D.M. & Reese, T.S. Arrays of particles in freeze-fractures of astrocytic membranes. J. Cell. Biol. 60, 316–320 (1974).
Frigeri, A. et al. Localization of MIWC and GLIP water channel homologs in neuromuscular, epithelial and glandular tissues. J. Cell. Sci. 108, 2993–3002 (1995).
Yang, B., Brown, D. & Verkman, A.S. The mercurial-insensitive water channel (AQP-4) form orthogonal arrays in stably transfected CHO cells. J. Biol. Chem. 271, 4577–4580 (1996).
Verbavatz, J.M., Ma, T., Gobin, R. & Verkman, A.S. Absence of orthogonal arrays in kidney, brain and muscle from transgenic knockout mice lacking water channel aquaporin-4. J. Cell Sci. 110, 2855–2860 (1997).
Rash, J.E., Yasumura, T., Hudson, C.S., Agre, P. & Nielsen, S. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membrane in rat brain and spinal cord. Proc. Natl. Acad. Sci. USA 95, 11981–11986 (1998).
Nagelhus, E.A. et al. Immunogold evidence suggests that coupling of K+ siphoning and water transport in rat retinal Muller cells is mediated by acoenrichment of Kir4.1 and AQP4 in specific membrane domains. Glia 26, 47–54 (1999).
Suzuki, M. et al. Disintegration of orthogonal arrays in perivascular astrocytic foot processes as an early event in acute global ischemia. Brain Res. 300, 141–145 (1984).
Hatton, J.D. & Ellisman, M.H. Orthogonal arrays are redistributed in the membranes of astroglia from alumina-induced epileptic foci. Epilepsia 25, 145–151 (1984).
Carrillo, P. et al. Prolonged severe hemorrhagic shock and resuscitation in rats does not cause subtle brain damage. J. Trauma 45, 239–248; discussion 248–239 (1998).
Fujimura, M. et al. Manganese superoxide dismutase mediates the early release of mitochondrial cytochrome C and subsequent DNA fragmentation after permanent focal cerebral ischemia in mice. J. Neurosci. 19, 3414–3422 (1999).
Yang, G. et al. Human copper-zinc superoxide dismutase transgenic mice are highly resistant to reperfusion injury after focal cerebral ischemia. Stroke 25, 165–170 (1994).
Swanson, R. A. et al. A semiautomated method for measuring brain infarct volume. J. Cereb. Blood Flow. Metab. 10, 290–293 (1990).
Acknowledgements
We thank R. Fishman and A. Marmarou for advice and critical discussions about edema models and quantitation of brain tissue water. We also thank. L. Qian for breeding and genotyping of transgenic mice and electron microscopy technologists J. Beck and M. Yoshimura. This work was supported by grants from the National Institutes of Health and National Cystic Fibrosis Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manley, G., Fujimura, M., Ma, T. et al. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med 6, 159–163 (2000). https://doi.org/10.1038/72256
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/72256
This article is cited by
-
MRI of cerebral oedema in ischaemic stroke and its current use in routine clinical practice
Neuroradiology (2024)
-
Alleviated cerebral infarction in male mice lacking all nitric oxide synthase isoforms after middle cerebral artery occlusion
Journal of Anesthesia (2024)
-
Aquaporins (AQPs) as a marker in the physiology of inflammation and its interaction studies with garcinol
Inflammopharmacology (2024)
-
Stroke and Neurogenesis: Bridging Clinical Observations to New Mechanistic Insights from Animal Models
Translational Stroke Research (2024)
-
Roles of neuropathology-associated reactive astrocytes: a systematic review
Acta Neuropathologica Communications (2023)