Abstract
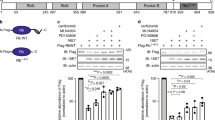
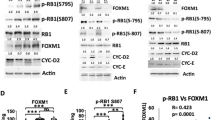
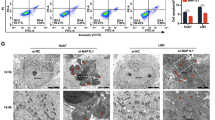
Hepatocellular carcinoma (HCC) is one of the most common cancers in Asia and Africa, where hepatitis virus infection and exposure to specific liver carcinogens are prevalent1,2. Although inactivation of some tumor suppressor genes such as p53 and p16INK4Ahas been identified3, no known oncogene is commonly activated in hepatocellular carcinomas. Here we have isolated genes overexpressed in hepatocellular carcinomas by cDNA subtractive hybridization4, and identified an oncoprotein consisting of six ankyrin repeats (gankyrin). The expression of gankyrin was increased in all 34 hepatocellular carcinomas studied. Gankyrin induced anchorage-independent growth and tumorigenicity in NIH/3T3 cells. Gankyrin bound to the product of the retinoblastoma gene (RB1), increasing its phosphorylation and releasing the activity of the transcription factor E2F-1. Gankyrin accelerated the degradation of RB1 in vitro and in vivo, and was identical to or interacted with a subunit of the 26S proteasome5,6. These results demonstrate the importance of ubiquitin–proteasome pathway in the regulation of cell growth and oncogenic transformation, and indicate that gankyrin overexpression contributes to hepatocarcinogenesis by destabilizing RB1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hsu, I.C., et al. Mutational hotspot in the p53 gene in human hepatocellular carcinomas . Nature 350, 427–428 (1991).
Bressac, B., Kew, M., Wands, J. & Ozturk, M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature 350, 429–431 ( 1991).
Liew, et al. High frequency of p16INK4A gene alterations in hepatocellular carcinoma . Oncogene 18, 789–795 (1999).
Higuchi, T., et al. Induction of tissue inhibitor of metalloproteinase 3 gene expression during in vitro decidualization of human endometrial stromal cells. Endocrinology 136, 4973– 4981 (1995).
Hori, T., et al. cDNA cloning and functional analysis of p28 (Nas6p) and p40.5 (Nas7p), two novel regulatory subunits of the 26S proteasome. Gene 216, 113–122 ( 1998).
Dawson, S., et al. The 26S-proteasome: regulation and substrate recognition. Mol. Biol. Rep. 24, 39–44 (1997).
Nevins, J.R., Leone, G., DeGregori, J. & Jakoi, L. Role of the Rb/E2F pathway in cell growth control. J. Cell. Physiol. 173 , 233–236 (1997).
Weinberg, R.A. the retinoblastoma protein and cell cycle control. Cell 81, 323–330 (1995).
Chen, Y., Sharp, Z.D. & Lee, W.H. HEC binds to the seventh regulatory subunitof the 26S proteasome and modulates the proteolysis of mitotic cyclins. J. Biol. Chem. 272, 24081–24087 (1997).
Mahaffey, D. & Rechsteiner, M. Discrimination between ubiquitin-dependent and ubiquitin-independent proteolytic pathways by the 26S proteasome subunit 5a. FEBS Lett. 450, 123–125 ( 1999).
Kisselev, A.F., Akopian, T., Woo, K.M. & Goldberg, A.L. The sizes of peptides generated from protein by mammalian 26 and 20 S proteasomes. Implications for understanding the degradative mechanism and antigen presentation. J. Biol. Chem. 274, 3363–3371 (1999).
Woitach, J.T., Zhang, M., Niu, C.H. & Thorgeirsson, S.S. A retinoblastoma- binding protein that affects cell-cycle control and confers transforming ability . Nature Genet. 19, 371– 374 (1998).
Johnson, D.G., Schneider-Broussard, R. Role of E2F in cell cycle control and cancer. Front. Biosci. 3, d447–448 (1998).
Israel, A. IkB kinase all zipped up. Nature 388, 519 –521 (1997).
Ciechanover, A., et al. The ubiquitin-mediated proteolytic system: involvement of molecular chaperones, degradation of oncoproteins, and activation of transcriptional regulators. Cold Spring Harb. Symp. Quant. Biol. 60 , 491–501 (1995).
Spataro, V., Norbury, C. & Harris, A.L. The ubiquitin-proteasome pathway in cancer. Br. J. Cancer 77, 448–455 (1998).
Scheffner, M., Huibregtse, J.M., Vierstra, R.D. & Howley, P.M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75, 495– 505 (1993).
Boyer, S.N., Wazer, D.E. & Band, V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 56, 4620–4624 (1996).
Berezutskaya, E. & Bagchi, S. The human papillomavirus E7 oncoprotein functionally interacts with the S4 subunit of the 26S proteasome . J. Biol. Chem. 272, 30135– 30140 (1997).
Smith-McCune, K., et al. Intranuclear localization of human papillomavirus 16 E7 during transformation and preferential binding of E7 to the Rb family member p130. Proc. Natl. Acad. Sci. USA 96, 6999–7004 (1999).
Acknowledgements
This work was supported in part by Grants-in-Aid from the Ministry of Science, Culture, Sports and Education of Japan, and by the European Union Biomedicine and Health Framework IV grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Higashitsuji, H., Itoh, K., Nagao, T. et al. Reduced stability of retinoblastoma protein by gankyrin, an oncogenic ankyrin-repeat protein overexpressed in hepatomas. Nat Med 6, 96–99 (2000). https://doi.org/10.1038/71600
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/71600
This article is cited by
-
ANKRD29, as a new prognostic and immunological biomarker of non–small cell lung cancer, inhibits cell growth and migration by regulating MAPK signaling pathway
Biology Direct (2023)
-
Gankyrin inhibits ferroptosis through the p53/SLC7A11/GPX4 axis in triple-negative breast cancer cells
Scientific Reports (2023)
-
Development and validation of a ubiquitin–proteasome system gene signature for prognostic prediction and immune microenvironment evaluation in hepatocellular carcinoma
Journal of Cancer Research and Clinical Oncology (2023)
-
Clinical implications of exosome-derived noncoding RNAs in liver
Laboratory Investigation (2022)
-
Gankyrin modulated non-small cell lung cancer progression via glycolysis metabolism in a YAP1-dependent manner
Cell Death Discovery (2022)