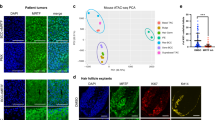
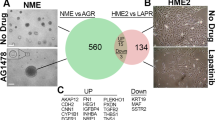
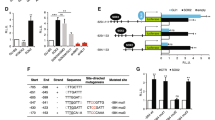
Abstract
Hedgehog pathway–dependent cancers can escape Smoothened (SMO) inhibition through mutations in genes encoding canonical hedgehog pathway components; however, around 50% of drug-resistant basal cell carcinomas (BCCs) lack additional variants of these genes. Here we use multidimensional genomics analysis of human and mouse drug-resistant BCCs to identify a noncanonical hedgehog activation pathway driven by the transcription factor serum response factor (SRF). Active SRF along with its coactivator megakaryoblastic leukemia 1 (MKL1) binds DNA near hedgehog target genes and forms a previously unknown protein complex with the hedgehog transcription factor glioma-associated oncogene family zinc finger-1 (GLI1), causing amplification of GLI1 transcriptional activity. We show that cytoskeletal activation through Rho and the formin family member Diaphanous (mDia) is required for SRF–MKL-driven GLI1 activation and for tumor cell viability. Remarkably, nuclear MKL1 staining served as a biomarker in tumors from mice and human subjects to predict tumor responsiveness to MKL inhibitors, highlighting the therapeutic potential of targeting this pathway. Thus, our study illuminates, for the first time, cytoskeletal-activation-driven transcription as a personalized therapeutic target for combatting drug-resistant malignancies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Junttila, M.R. & de Sauvage, F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 501, 346–354 (2013).
Ransohoff, K.J., Tang, J.Y. & Sarin, K.Y. Squamous change in basal-cell carcinoma with drug resistance. N. Engl. J. Med. 373, 1079–1082 (2015).
Atwood, S.X., Whitson, R.J. & Oro, A.E. Advanced treatment for basal cell carcinomas. Cold Spring Harb. Perspect. Med. 4, a013581 (2014).
Amaral, L. et al. Inhibitors of bacterial efflux pumps that also inhibit efflux pumps of cancer cells. Anticancer Res. 32, 2947–2957 (2012).
Thomas-Schoemann, A. et al. Drug interactions with solid tumour–targeted therapies. Crit. Rev. Oncol. Hematol. 89, 179–196 (2014).
Nürnberg, A., Kitzing, T. & Grosse, R. Nucleating actin for invasion. Nat. Rev. Cancer 11, 177–187 (2011).
Barker, H.E., Paget, J.T., Khan, A.A. & Harrington, K.J. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat. Rev. Cancer 15, 409–425 (2015).
McMillin, D.W., Negri, J.M. & Mitsiades, C.S. The role of tumour–stromal interactions in modifying drug response: challenges and opportunities. Nat. Rev. Drug Discov. 12, 217–228 (2013).
Basset-Seguin, N., Sharpe, H.J. & de Sauvage, F.J. Efficacy of hedgehog pathway inhibitors in basal cell carcinoma. Mol. Cancer Ther. 14, 633–641 (2015).
Sekulic, A. et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 366, 2171–2179 (2012).
Chang, A.L. & Oro, A.E. Initial assessment of tumor regrowth after vismodegib in advanced basal cell carcinoma. Arch. Dermatol. 148, 1324–1325 (2012).
Atwood, S.X. et al. Smoothened variants explain the majority of drug resistance in basal cell carcinoma. Cancer Cell 27, 342–353 (2015).
Sharpe, H.J. et al. Genomic analysis of smoothened inhibitor resistance in basal cell carcinoma. Cancer Cell 27, 327–341 (2015).
Atwood, S.X., Li, M., Lee, A., Tang, J.Y. & Oro, A.E. GLI activation by atypical protein kinase C ι/λ regulates the growth of basal cell carcinomas. Nature 494, 484–488 (2013).
Atwood, S.X. et al. Rolling the genetic dice: neutral and deleterious smoothened mutations in drug-resistant basal cell carcinoma. J. Invest. Dermatol. 135, 2138–2141 (2015).
Zaromytidou, A.I., Miralles, F. & Treisman, R. MAL and ternary complex factor use different mechanisms to contact a common surface on the serum response factor DNA-binding domain. Mol. Cell. Biol. 26, 4134–4148 (2006).
Wang, Z. et al. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature 428, 185–189 (2004).
Marais, R., Wynne, J. & Treisman, R. The SRF accessory protein Elk-1 contains a growth factor–regulated transcriptional activation domain. Cell 73, 381–393 (1993).
Janknecht, R. & Nordheim, A. Elk-1 protein domains required for direct and SRF-assisted DNA-binding. Nucleic Acids Res. 20, 3317–3324 (1992).
Miralles, F., Posern, G., Zaromytidou, A.I. & Treisman, R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 113, 329–342 (2003).
Wang, G.Y. et al. Establishment of murine basal cell carcinoma allografts: a potential model for preclinical drug testing and for molecular analysis. J. Invest. Dermatol. 131, 2298–2305 (2011).
Webster, D.E. et al. Enhancer-targeted genome editing selectively blocks innate resistance to oncokinase inhibition. Genome Res. 24, 751–760 (2014).
Bonilla, X. et al. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat. Genet. 48, 398–406 (2016).
Olson, E.N. & Nordheim, A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nat. Rev. Mol. Cell Biol. 11, 353–365 (2010).
Heidenreich, O. et al. MAPKAP kinase 2 phosphorylates serum response factor in vitro and in vivo. J. Biol. Chem. 274, 14434–14443 (1999).
Zhang, H.M. et al. Mitogen-induced recruitment of ERK and MSK to SRE promoter complexes by ternary complex factor Elk-1. Nucleic Acids Res. 36, 2594–2607 (2008).
Blaker, A.L., Taylor, J.M. & Mack, C.P. PKA-dependent phosphorylation of serum response factor inhibits smooth muscle–specific gene expression. Arterioscler. Thromb. Vasc. Biol. 29, 2153–2160 (2009).
Pawłowski, R., Rajakylä, E.K., Vartiainen, M.K. & Treisman, R. An actin-regulated importin α/β-dependent extended bipartite NLS directs nuclear import of MRTF-A. EMBO J. 29, 3448–3458 (2010).
Mokady, D. & Meiri, D. RhoGTPases—a novel link between cytoskeleton organization and cisplatin resistance. Drug Resist. Updat. 19, 22–32 (2015).
Guettler, S. et al. RPEL motifs link the serum response factor cofactor MAL but not myocardin to Rho signaling via actin binding. Mol. Cell. Biol. 28, 732–742 (2008).
Li, F. & Higgs, H.N. The mouse Formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr. Biol. 13, 1335–1340 (2003).
Rath, N. & Olson, M.F. Rho-associated kinases in tumorigenesis: re-considering ROCK inhibition for cancer therapy. EMBO Rep. 13, 900–908 (2012).
Haak, A.J. et al. Targeting the myofibroblast genetic switch: inhibitors of myocardin-related transcription factor/serum response factor–regulated gene transcription prevent fibrosis in a murine model of skin injury. J. Pharmacol. Exp. Ther. 349, 480–486 (2014).
Sisson, T.H. et al. Inhibition of myocardin-related transcription factor/serum response factor signaling decreases lung fibrosis and promotes mesenchymal cell apoptosis. Am. J. Pathol. 185, 969–986 (2015).
Ally, M.S. et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 152, 452–456 (2016).
Sekulic, A. et al. Pivotal ERIVANCE basal cell carcinoma (BCC) study: 12-month update of efficacy and safety of vismodegib in advanced BCC. J. Am. Acad. Dermatol. 72, 1021–1026. e8 (2015).
Vasudevan, H.N. & Soriano, P. SRF regulates craniofacial development through selective recruitment of MRTF cofactors by PDGF signaling. Dev. Cell 31, 332–344 (2014).
Esnault, C. et al. Rho–actin signaling to the MRTF coactivators dominates the immediate transcriptional response to serum in fibroblasts. Genes Dev. 28, 943–958 (2014).
Schneider, P. et al. Identification of a novel actin-dependent signal transducing module allows for the targeted degradation of GLI1. Nat. Commun. 6, 8023 (2015).
Horn, A. et al. Hedgehog signaling controls fibroblast activation and tissue fibrosis in systemic sclerosis. Arthritis Rheum. 64, 2724–2733 (2012).
Parri, M. & Chiarugi, P. Rac and Rho GTPases in cancer cell motility control. Cell Commun. Signal 8, 23 (2010).
Jiang, P., Enomoto, A. & Takahashi, M. Cell biology of the movement of breast cancer cells: intracellular signalling and the actin cytoskeleton. Cancer Lett. 284, 122–130 (2009).
Morita, K., Lo Celso, C., Spencer-Dene, B., Zouboulis, C.C. & Watt, F.M. HAN11 binds mDia1 and controls GLI1 transcriptional activity. J. Dermatol. Sci. 44, 11–20 (2006).
Johnson, L.A. et al. Novel Rho/MRTF/SRF inhibitors block matrix-stiffness and TGF-β-induced fibrogenesis in human colonic myofibroblasts. Inflamm. Bowel Dis. 20, 154–165 (2014).
Wang, G.Y., Wang, J., Mancianti, M.L. & Epstein, E.H. Jr. Basal cell carcinomas arise from hair follicle stem cells in Ptch1+/− mice. Cancer Cell 19, 114–124 (2011).
Kuleshov, M.V. et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44, W90–W97 (2016).
Peterson, K.A. et al. Neural-specific Sox2 input and differential Gli-binding affinity provide context and positional information in Shh-directed neural patterning. Genes Dev. 26, 2802–2816 (2012).
Lee, E.Y. et al. Hedgehog pathway–regulated gene networks in cerebellum development and tumorigenesis. Proc. Natl. Acad. Sci. USA 107, 9736–9741 (2010).
Vokes, S.A., Ji, H., Wong, W.H. & McMahon, A.P. A genome-scale analysis of the cis-regulatory circuitry underlying sonic hedgehog–mediated patterning of the mammalian limb. Genes Dev. 22, 2651–2663 (2008).
Sullivan, A.L. et al. Serum response factor utilizes distinct promoter- and enhancer-based mechanisms to regulate cytoskeletal gene expression in macrophages. Mol. Cell. Biol. 31, 861–875 (2011).
McLean, C.Y. et al. GREAT improves functional interpretation of cis-regulatory regions. Nat. Biotechnol. 28, 495–501 (2010).
Noubissi, F.K. et al. Role of CRD-BP in the growth of human basal cell carcinoma cells. J. Invest. Dermatol. 134, 1718–1724 (2014).
Acknowledgements
The authors wish to thank all members of the laboratory of A.E.O. and the Stanford Dermatology Department for suggestions and guidance. Specifically, the authors would like to thank S.P. Melo for guidance and assistance with ChIP–seq analyses. This work was funded by the V Foundation Translational Award, National Cancer Institute (R01CA157895), the National Institute of Arthritis and Musculoskeletal Disease (R01AR04786 and 5ARO54780), the Stanford Epithelial Biology Training Grant award to R.J.W. (T32-AR007422), National Institutes of Health (NIH) Pathway to Independence Award to S.X.A. (4R00CA17684703), and a Damon Runyon clinical investigatory award (J.Y.T.). The Stanford Cell Sciences Imaging Facility provided instrumentation and technical assistance for microscopy using a Leica SP8 confocal microscope funded by a National Center for Research Resources grant (1S10OD010580). The Stanford functional Genomics Facility provided sequencing services for ASZ RNA-seq and SRF ChIP–seq using the Illumina HiSeq 4000 platform purchased using an NIH S10 Shared Instrumentation Grant (S10OD018220).
Author information
Authors and Affiliations
Contributions
R.J.W. and A.E.O. designed the experiments and wrote the manuscript. R.J.W. performed the majority of experiments. A.L. performed all mouse tumor generation experiments except for rBCC drug treatment experiments with vismodegib and CCG-203971, which were administered by R.J.W. CCG-203971 in vivo drug treatment was repeated by M.A.F. The majority of cellular and molecular experiments were assisted and optimized by N.M.U. Exome- and RNA-seq analyses were carried out by J.R.L. and G.S. Co-IP experiments were carried out by A.M. C.Y.Y. assisted with SRF and MKL1 inhibition studies and knockdown studies as well as RHO and mDia experiments. S.X.A. assisted with RNA- and exome-seq library generation. S.Z.A., S.T.H., and K.Y.S. provided human tumor samples and annotation. E.H.E. and J.Y.T. designed the PTC53-BCC mouse resistance model. A.S.B., M.P.S., and E.Y.L. provided GLI ChIP data.
Corresponding author
Ethics declarations
Competing interests
A.E.O. is a clinical investigator funded by Novartis.
Supplementary information
Supplementary Figures
Supplementary Figures 1–28 (PDF 5789 kb)
Rights and permissions
About this article
Cite this article
Whitson, R., Lee, A., Urman, N. et al. Noncanonical hedgehog pathway activation through SRF–MKL1 promotes drug resistance in basal cell carcinomas. Nat Med 24, 271–281 (2018). https://doi.org/10.1038/nm.4476
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4476
This article is cited by
-
Skin basal cell carcinomas assemble a pro-tumorigenic spatially organized and self-propagating Trem2+ myeloid niche
Nature Communications (2023)
-
Hedgehog signaling in tissue homeostasis, cancers, and targeted therapies
Signal Transduction and Targeted Therapy (2023)
-
Coordination of canonical and noncanonical Hedgehog signalling pathways mediated by WDR11 during primordial germ cell development
Scientific Reports (2023)
-
The role of Hedgehog and Notch signaling pathway in cancer
Molecular Biomedicine (2022)
-
LPA receptor 1 (LPAR1) is a novel interaction partner of Filamin A that promotes Filamin A phosphorylation, MRTF-A transcriptional activity and oncogene-induced senescence
Oncogenesis (2022)