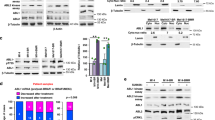
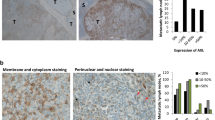
Abstract
Intratumor heterogeneity is a key factor contributing to therapeutic failure and, hence, cancer lethality. Heterogeneous tumors show partial therapy responses, allowing for the emergence of drug-resistant clones that often express high levels of the receptor tyrosine kinase AXL. In melanoma, AXL-high cells are resistant to MAPK pathway inhibitors, whereas AXL-low cells are sensitive to these inhibitors, rationalizing a differential therapeutic approach. We developed an antibody-drug conjugate, AXL-107-MMAE, comprising a human AXL antibody linked to the microtubule-disrupting agent monomethyl auristatin E. We found that AXL-107-MMAE, as a single agent, displayed potent in vivo anti-tumor activity in patient-derived xenografts, including melanoma, lung, pancreas and cervical cancer. By eliminating distinct populations in heterogeneous melanoma cell pools, AXL-107-MMAE and MAPK pathway inhibitors cooperatively inhibited tumor growth. Furthermore, by inducing AXL transcription, BRAF/MEK inhibitors potentiated the efficacy of AXL-107-MMAE. These findings provide proof of concept for the premise that rationalized combinatorial targeting of distinct populations in heterogeneous tumors may improve therapeutic effect, and merit clinical validation of AXL-107-MMAE in both treatment-naive and drug-resistant cancers in mono- or combination therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
McGranahan, N. & Swanton, C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell 168, 613–628 (2017).
Aparicio, S. & Caldas, C. The implications of clonal genome evolution for cancer medicine. N. Engl. J. Med. 368, 842–851 (2013).
Sharma, P., Hu-Lieskovan, S., Wargo, J.A. & Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 (2017).
Diaz, L.A. Jr. et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 486, 537–540 (2012).
Long, G.V. et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N. Engl. J. Med. 371, 1877–1888 (2014).
Robert, C. et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 372, 30–39 (2015).
Larkin, J. et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 371, 1867–1876 (2014).
Flaherty, K.T. et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N. Engl. J. Med. 367, 1694–1703 (2012).
Chapman, P.B. et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 (2011).
Long, G.V. et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol. 17, 1743–1754 (2016).
Smith, M.P. et al. The immune microenvironment confers resistance to MAPK pathway inhibitors through macrophage-derived TNFα. Cancer Discov. 4, 1214–1229 (2014).
Nazarian, R. et al. Melanomas acquire resistance to B-RAFV600E inhibition by RTK or N-RAS upregulation. Nature 468, 973–977 (2010).
Girotti, M.R., Saturno, G., Lorigan, P. & Marais, R. No longer an untreatable disease: how targeted and immunotherapies have changed the management of melanoma patients. Mol. Oncol. 8, 1140–1158 (2014).
Davies, M.A. & Samuels, Y. Analysis of the genome to personalize therapy for melanoma. Oncogene 29, 5545–5555 (2010).
Shi, H. et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 4, 80–93 (2014).
Kemper, K. et al. Intra- and inter-tumor heterogeneity in a vemurafenib-resistant melanoma patient and derived xenografts. EMBO Mol. Med. 7, 1104–1118 (2015).
Müller, J. et al. Low MITF/AXL ratio predicts early resistance to multiple targeted drugs in melanoma. Nat. Commun. 5, 5712 (2014).
Konieczkowski, D.J. et al. A melanoma cell state distinction influences sensitivity to MAPK pathway inhibitors. Cancer Discov. 4, 816–827 (2014).
Leucci, E., Close, P. & Marine, J.-C. Translation rewiring at the heart of phenotype switching in melanoma. Pigment Cell Melanoma Res. 30, 282–283 (2017).
Hoek, K.S. et al. Metastatic potential of melanomas defined by specific gene expression profiles with no BRAF signature. Pigment Cell Res. 19, 290–302 (2006).
Hoek, K.S. et al. In vivo switching of human melanoma cells between proliferative and invasive states. Cancer Res. 68, 650–656 (2008).
Elkabets, M. et al. AXL mediates resistance to PI3Kα inhibition by activating the EGFR/PKC/mTOR axis in head and neck and esophageal squamous cell carcinomas. Cancer Cell 27, 533–546 (2015).
Reichl, P. et al. Axl activates autocrine transforming growth factor-β signaling in hepatocellular carcinoma. Hepatology 61, 930–941 (2015).
Bansal, N., Mishra, P.J., Stein, M., DiPaola, R.S. & Bertino, J.R. Axl receptor tyrosine kinase is up-regulated in metformin resistant prostate cancer cells. Oncotarget 6, 15321–15331 (2015).
Byers, L.A. et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin. Cancer Res. 19, 279–290 (2013).
Zhang, Z. et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat. Genet. 44, 852–860 (2012).
Creedon, H. et al. Exploring mechanisms of acquired resistance to HER2 (human epidermal growth factor receptor 2)-targeted therapies in breast cancer. Biochem. Soc. Trans. 42, 822–830 (2014).
Zhou, L. et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene 35, 2687–2697 (2016).
Debruyne, D.N. et al. ALK inhibitor resistance in ALKF1174L-driven neuroblastoma is associated with AXL activation and induction of EMT. Oncogene 35, 3681–3691 (2016).
Wang, C. et al. Gas6/Axl axis contributes to chemoresistance and metastasis in breast cancer through Akt/GSK-3β/β-catenin signaling. Theranostics 6, 1205–1219 (2016).
Kurokawa, M., Ise, N., Omi, K., Goishi, K. & Higashiyama, S. Cisplatin influences acquisition of resistance to molecular-targeted agents through epithelial-mesenchymal transition-like changes. Cancer Sci. 104, 904–911 (2013).
Hong, C.-C. et al. Receptor tyrosine kinase AXL is induced by chemotherapy drugs and overexpression of AXL confers drug resistance in acute myeloid leukemia. Cancer Lett. 268, 314–324 (2008).
Aguilera, T.A. et al. Reprogramming the immunological microenvironment through radiation and targeting Axl. Nat. Commun. 7, 13898 (2016).
Bhang, H.-E.C. et al. Studying clonal dynamics in response to cancer therapy using high-complexity barcoding. Nat. Med. 21, 440–448 (2015).
Hata, A.N. et al. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat. Med. 22, 262–269 (2016).
Sharma, S.V. et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 141, 69–80 (2010).
Turke, A.B. et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell 17, 77–88 (2010).
Cichoń, M.A. et al. The receptor tyrosine kinase Axl regulates cell-cell adhesion and stemness in cutaneous squamous cell carcinoma. Oncogene 33, 4185–4192 (2014).
Mak, M.P. et al. A patient-derived, pan-cancer EMT signature identifies global molecular alterations and immune target enrichment following epithelial-to-mesenchymal transition. Clin. Cancer Res. 22, 609–620 (2016).
Garnett, M.J. et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 483, 570–575 (2012).
Loges, S. et al. Malignant cells fuel tumor growth by educating infiltrating leukocytes to produce the mitogen Gas6. Blood 115, 2264–2273 (2010).
Adams, G.P. et al. High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res. 61, 4750–4755 (2001).
Donaghy, H. Effects of antibody, drug and linker on the preclinical and clinical toxicities of antibody-drug conjugates. MAbs 8, 659–671 (2016).
Chen, R. et al. CD30 downregulation, MMAE resistance, and MDR1 upregulation are all associated with resistance to brentuximab vedotin. Mol. Cancer Ther. 14, 1376–1384 (2015).
Szakács, G., Paterson, J.K., Ludwig, J.A., Booth-Genthe, C. & Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 5, 219–234 (2006).
Holderfield, M., Deuker, M.M., McCormick, F. & McMahon, M. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat. Rev. Cancer 14, 455–467 (2014).
Kemper, K. et al. BRAFV600E kinase domain duplication identified in therapy-refractory melanoma patient-derived xenografts. Cell Rep. 16, 263–277 (2016).
de Goeij, B.E. & Lambert, J.M. New developments for antibody-drug conjugate–based therapeutic approaches. Curr. Opin. Immunol. 40, 14–23 (2016).
Thomas, A., Teicher, B.A. & Hassan, R. Antibody-drug conjugates for cancer therapy. Lancet Oncol. 17, e254–e262 (2016).
Asundi, J. et al. MAPK pathway inhibition enhances the efficacy of an anti–endothelin B receptor drug conjugate by inducing target expression in melanoma. Mol. Cancer Ther. 13, 1599–1610 (2014).
Breij, E.C.W. et al. An antibody-drug conjugate that targets tissue factor exhibits potent therapeutic activity against a broad range of solid tumors. Cancer Res. 74, 1214–1226 (2014).
Doronina, S.O. et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat. Biotechnol. 21, 778–784 (2003).
Prahallad, A. et al. Unresponsiveness of colon cancer to BRAFV600E inhibition through feedback activation of EGFR. Nature 483, 100–103 (2012).
Manchado, E. et al. A combinatorial strategy for treating KRAS-mutant lung cancer. Nature 534, 647–651 (2016).
Miller, M.A. et al. Reduced proteolytic shedding of receptor tyrosine kinases is a post-translational mechanism of kinase inhibitor resistance. Cancer Discov. 6, 382–399 (2016).
Creedon, H. et al. Identification of novel pathways linking epithelial-to-mesenchymal transition with resistance to HER2-targeted therapy. Oncotarget 7, 11539–11552 (2016).
Xu, W. et al. Up-regulation of the Hippo pathway effector TAZ renders lung adenocarcinoma cells harboring EGFR-T790M mutation resistant to gefitinib. Cell Biosci. 5, 7 (2015).
Tirosh, I. et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352, 189–196 (2016).
Shaffer, S.M. et al. Rare cell variability and drug-induced reprogramming as a mode of cancer drug resistance. Nature 546, 431–435 (2017).
Fishwild, D.M. et al. High-avidity human IgG κ monoclonal antibodies from a novel strain of minilocus transgenic mice. Nat. Biotechnol. 14, 845–851 (1996).
Vink, T., Oudshoorn-Dickmann, M., Roza, M., Reitsma, J.-J. & de Jong, R.N. A simple, robust and highly efficient transient expression system for producing antibodies. Methods 65, 5–10 (2014).
Burton, D.R. et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266, 1024–1027 (1994).
Colaprico, A. et al. TCGAbiolinks: an R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 44, e71 (2016).
Acknowledgements
We thank all the members of the Peeper and Blank laboratories for their valuable input and the FACS facility and animal facility at the NKI for their support. We would like to acknowledge the NKI-AVL Core Facility Molecular Pathology & Biobanking (CFMPB) for supplying NKI-AVL Biobank material and lab support. We thank M. van der Ven and the intervention unit (NKI) for their help with animal experiments, M. Mertz and E. Gielen for their help with confocal microscopy, and H. Witteveen, M. Houtkamp, G. Rigter and T. Kroes for help with experiments. The research leading to these results has been funded by a grant from Genmab, by the European Research Council under the European Union′s Seventh Framework Programme (FP7/2007-2013)/ERC synergy grant agreement n° 319661 COMBATCANCER and grants NKI 2014-7241, NKI 2013-5799 and NKI 2017-10425 from the Dutch Cancer Society (KWF).
Author information
Authors and Affiliations
Contributions
J.B., L.A.K., E.C.W.B., M.L.J., D.S., D.S.P. and P.W.H.I.P. designed the study, analyzed the data and wrote the manuscript. J.B., M.A.L., D.W.V., N.P. and E.G.-v.d.H. performed the experiments. O.K. performed the bioinformatics analyses. K.K. and D.S.P. developed the melanoma PDX-platform and engineered PDX-derived cell lines. A.S. and J.B. performed in vivo melanoma experiments. J.-Y.S. analyzed IHC from in vivo experiments. T.K. and C.U.B. gave critical input. J.T.M. directed toxicological analyses. M.G.F. and E.A.R. provided paired human melanoma samples. D.S.P. and P.W.H.I.P. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
L.K., E.B., E.G.-v.d.H., N.P., M.J. and D.S. are full-time Genmab employees and have warrants and/or stock. P.P. is a Genmab shareholder. C.U.B. receives grants and/or research support from Novartis and BMS, and has received honoraria or consultation fees for MSD, BMS, Roche, Novartis, GSK, Pfizer and Lilly, and is a stock shareholder of Verastem.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 and Supplementary Tables 1–4 (PDF 16466 kb)
Rights and permissions
About this article
Cite this article
Boshuizen, J., Koopman, L., Krijgsman, O. et al. Cooperative targeting of melanoma heterogeneity with an AXL antibody-drug conjugate and BRAF/MEK inhibitors. Nat Med 24, 203–212 (2018). https://doi.org/10.1038/nm.4472
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4472
This article is cited by
-
The promise and challenges of combination therapies with antibody-drug conjugates in solid tumors
Journal of Hematology & Oncology (2024)
-
Drug conjugates for the treatment of lung cancer: from drug discovery to clinical practice
Experimental Hematology & Oncology (2024)
-
Simultaneous targeted and discovery-driven clinical proteotyping using hybrid-PRM/DIA
Clinical Proteomics (2024)
-
Therapeutic targeting of the functionally elusive TAM receptor family
Nature Reviews Drug Discovery (2024)
-
Modeling stress-induced responses: plasticity in continuous state space and gradual clonal evolution
Theory in Biosciences (2024)