Abstract
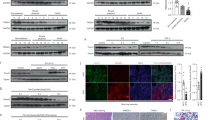
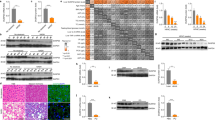
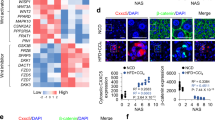
Activation of apoptosis signal-regulating kinase 1 (ASK1) in hepatocytes is a key process in the progression of nonalcoholic steatohepatitis (NASH) and a promising target for treatment of the condition. However, the mechanism underlying ASK1 activation is still unclear, and thus the endogenous regulators of this kinase remain open to be exploited as potential therapeutic targets. In screening for proteins that interact with ASK1 in the context of NASH, we identified the deubiquitinase tumor necrosis factor alpha–induced protein 3 (TNFAIP3) as a key endogenous suppressor of ASK1 activation, and we found that TNFAIP3 directly interacts with and deubiquitinates ASK1 in hepatocytes. Hepatocyte-specific ablation of Tnfaip3 exacerbated nonalcoholic fatty liver disease– and NASH-related phenotypes in mice, including glucose metabolism disorders, lipid accumulation and enhanced inflammation, in an ASK1-dependent manner. In contrast, transgenic or adeno-associated virus–mediated TNFAIP3 gene delivery in the liver in both mouse and nonhuman primate models of NASH substantially blocked the onset and progression of the disease. These results implicate TNFAIP3 as a functionally important endogenous suppressor of ASK1 hyperactivation in the pathogenesis of NASH and identify it as a potential new molecular target for NASH therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Byrne, C.D. & Targher, G. NAFLD: a multisystem disease. J. Hepatol. 62 (Suppl.), S47–S64 (2015).
Michelotti, G.A., Machado, M.V. & Diehl, A.M. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 10, 656–665 (2013).
Villanueva, M.T. Liver disease: conscious uncoupling in NASH. Nat. Rev. Drug Discov. 16, 238–239 (2017).
Targher, G., Day, C.P. & Bonora, E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N. Engl. J. Med. 363, 1341–1350 (2010).
Czech, M.P. Obesity notches up fatty liver. Nat. Med. 19, 969–971 (2013).
Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006).
Gregor, M.F. & Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445 (2011).
Xie, L. et al. DKK3 expression in hepatocytes defines susceptibility to liver steatosis and obesity. J. Hepatol. 65, 113–124 (2016).
Wang, P.X. et al. Targeting CASP8 and FADD-like apoptosis regulator ameliorates nonalcoholic steatohepatitis in mice and nonhuman primates. Nat. Med. 23, 439–449 (2017).
Xiang, M. et al. Targeting hepatic TRAF1–ASK1 signaling to improve inflammation, insulin resistance, and hepatic steatosis. J. Hepatol. 64, 1365–1377 (2016).
Vernia, S. et al. The PPARα–FGF21 hormone axis contributes to metabolic regulation by the hepatic JNK signaling pathway. Cell Metab. 20, 512–525 (2014).
Lawan, A. et al. Hepatic mitogen-activated protein kinase phosphatase 1 selectively regulates glucose metabolism and energy homeostasis. Mol. Cell. Biol. 35, 26–40 (2015).
Li, P. et al. LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat. Med. 21, 239–247 (2015).
Sabio, G. et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 322, 1539–1543 (2008).
Lin, J.H., Zhang, J.J., Lin, S.L. & Chertow, G.M. Design of a phase 2 clinical trial of an ASK1 inhibitor, GS-4997, in patients with diabetic kidney disease. Nephron 129, 29–33 (2015).
Won, M. et al. Novel anti-apoptotic mechanism of A20 through targeting ASK1 to suppress TNF-induced JNK activation. Cell Death Differ. 17, 1830–1841 (2010).
Nishida, T., Hattori, K. & Watanabe, K. The regulatory and signaling mechanisms of the ASK family. Adv. Biol. Regul. S2212-4926(17)30119-7 (2017).
Chen, M.B. et al. Activation of AMP-activated protein kinase (AMPK) mediates plumbagin-induced apoptosis and growth inhibition in cultured human colon cancer cells. Cell. Signal. 25, 1993–2002 (2013).
Boronkai, A. et al. Potentiation of paclitaxel-induced apoptosis by galectin-13 overexpression via activation of Ask-1–p38-MAP kinase and JNK/SAPK pathways and suppression of Akt and ERK1/2 activation in U-937 human macrophage cells. Eur. J. Cell Biol. 88, 753–763 (2009).
Saitoh, M. et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 17, 2596–2606 (1998).
Petrvalska, O. et al. Structural insight into the 14-3-3 protein–dependent inhibition of protein kinase ASK1 (apoptosis signal-regulating kinase 1). J. Biol. Chem. 291, 20753–20765 (2016).
Yu, Z. et al. Lys29-linkage of ASK1 by Skp1–Cullin 1–Fbxo21 ubiquitin ligase complex is required for antiviral innate response. eLife 5, e14087 (2016).
Lu, T.T. et al. Dimerization and ubiquitin mediated recruitment of A20, a complex deubiquitinating enzyme. Immunity 38, 896–905 (2013).
Wertz, I.E. et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature 430, 694–699 (2004).
Bosanac, I. et al. Ubiquitin binding to A20 ZnF4 is required for modulation of NF-κB signaling. Mol. Cell 40, 548–557 (2010).
Maruyama, H., Kiyono, S., Kondo, T., Sekimoto, T. & Yokosuka, O. Palmitate-induced regulation of PPARγ via PGC1α: a mechanism for lipid accumulation in the liver in nonalcoholic fatty liver disease. Int. J. Med. Sci. 13, 169–178 (2016).
Feng, J. et al. miR-21 attenuates lipopolysaccharide-induced lipid accumulation and inflammatory response: potential role in cerebrovascular disease. Lipids Health Dis. 13, 27 (2014).
McCommis, K.S. et al. Targeting the mitochondrial pyruvate carrier attenuates fibrosis in a mouse model of nonalcoholic steatohepatitis. Hepatology 65, 1543–1556 (2017).
Ye, D. et al. Lipocalin-2 mediates non-alcoholic steatohepatitis by promoting neutrophil–macrophage crosstalk via the induction of CXCR2. J. Hepatol. 65, 988–997 (2016).
Nam, H.J. et al. Structure of adeno-associated virus serotype 8, a gene therapy vector. J. Virol. 81, 12260–12271 (2007).
Tobiume, K. et al. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2, 222–228 (2001).
Weijman, J.F. et al. Structural basis of autoregulatory scaffolding by apoptosis signal-regulating kinase 1. Proc. Natl. Acad. Sci. USA 114, E2096–E2105 (2017).
Geleziunas, R., Xu, W., Takeda, K., Ichijo, H. & Greene, W.C. HIV-1 Nef inhibits ASK1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature 410, 834–838 (2001).
Matsuzawa, A. et al. ROS-dependent activation of the TRAF6–ASK1–p38 pathway is selectively required for TLR4-mediated innate immunity. Nat. Immunol. 6, 587–592 (2005).
Okazaki, T. et al. The ASK family kinases differentially mediate induction of type I interferon and apoptosis during the antiviral response. Sci. Signal. 8, ra78 (2015).
Huang, Q. et al. Thioredoxin-2 inhibits mitochondrial reactive oxygen species generation and apoptosis stress kinase-1 activity to maintain cardiac function. Circulation 131, 1082–1097 (2015).
Zhang, Y. et al. Dickkopf-3 attenuates pressure overload–induced cardiac remodelling. Cardiovasc. Res. 102, 35–45 (2014).
Hayakawa, Y. et al. Apoptosis signal-regulating kinase 1 and cyclin D1 compose a positive feedback loop contributing to tumor growth in gastric cancer. Proc. Natl. Acad. Sci. USA 108, 780–785 (2011).
Stark, M.S. et al. Frequent somatic mutations in MAP3K5 and MAP3K9 in metastatic melanoma identified by exome sequencing. Nat. Genet. 44, 165–169 (2011).
Tobiume, K., Saitoh, M. & Ichijo, H. Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. J. Cell. Physiol. 191, 95–104 (2002).
Noguchi, T. et al. Recruitment of tumor necrosis factor receptor–associated factor family proteins to apoptosis signal-regulating kinase 1 signalosome is essential for oxidative stress–induced cell death. J. Biol. Chem. 280, 37033–37040 (2005).
He, Y., Zhang, W., Zhang, R., Zhang, H. & Min, W. SOCS1 inhibits tumor necrosis factor–induced activation of ASK1–JNK inflammatory signaling by mediating ASK1 degradation. J. Biol. Chem. 281, 5559–5566 (2006).
Zhao, Y., Conze, D.B., Hanover, J.A. & Ashwell, J.D. Tumor necrosis factor receptor 2 signaling induces selective c-IAP1-dependent ASK1 ubiquitination and terminates mitogen-activated protein kinase signaling. J. Biol. Chem. 282, 7777–7782 (2007).
Maruyama, T. et al. Roquin-2 promotes ubiquitin-mediated degradation of ASK1 to regulate stress responses. Sci. Signal. 7, ra8 (2014).
Lee, E.G. et al. Failure to regulate TNF-induced NF-κB and cell death responses in A20-deficient mice. Science 289, 2350–2354 (2000).
Onizawa, M. et al. The ubiquitin-modifying enzyme A20 restricts ubiquitination of the kinase RIPK3 and protects cells from necroptosis. Nat. Immunol. 16, 618–627 (2015).
Ma, A. & Malynn, B.A. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat. Rev. Immunol. 12, 774–785 (2012).
Shembade, N., Ma, A. & Harhaj, E.W. Inhibition of NF-κB signaling by A20 through disruption of ubiquitin enzyme complexes. Science 327, 1135–1139 (2010).
Turer, E.E. et al. Homeostatic MyD88-dependent signals cause lethal inflammation in the absence of A20. J. Exp. Med. 205, 451–464 (2008).
Boone, D.L. et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 5, 1052–1060 (2004).
Coornaert, B., Carpentier, I. & Beyaert, R. A20: central gatekeeper in inflammation and immunity. J. Biol. Chem. 284, 8217–8221 (2009).
Malynn, B.A. & Ma, A. A20 takes on tumors: tumor suppression by an ubiquitin-editing enzyme. J. Exp. Med. 206, 977–980 (2009).
Studer, P. et al. Significant lethality following liver resection in A20 heterozygous knockout mice uncovers a key role for A20 in liver regeneration. Cell Death Differ. 22, 2068–2077 (2015).
Catrysse, L. et al. A20 prevents chronic liver inflammation and cancer by protecting hepatocytes from death. Cell Death Dis. 7, e2250 (2016).
Ai, L. et al. A20 attenuates FFAs-induced lipid accumulation in nonalcoholic steatohepatitis. Int. J. Biol. Sci. 11, 1436–1446 (2015).
Ramsey, H.E. et al. A20 protects mice from lethal liver ischemia/reperfusion injury by increasing peroxisome proliferator-activated receptor-α expression. Liver Transpl. 15, 1613–1621 (2009).
Longo, C.R. et al. A20 protects mice from lethal radical hepatectomy by promoting hepatocyte proliferation via a p21waf1-dependent mechanism. Hepatology 42, 156–164 (2005).
Huang, H. et al. Tumor suppressor A20 protects against cardiac hypertrophy and fibrosis by blocking transforming growth factor-beta-activated kinase 1-dependent signaling. Hypertension 56, 232–239 (2010).
Li, H.L. et al. Targeted cardiac overexpression of A20 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circulation 115, 1885–1894 (2007).
Catrysse, L., Vereecke, L., Beyaert, R. & van Loo, G. A20 in inflammation and autoimmunity. Trends Immunol. 35, 22–31 (2014).
Deng, K.Q. et al. Suppressor of IKK is an essential negative regulator of pathological cardiac hypertrophy. Nat. Commun. 7, 11432 (2016).
Zhao, G.N. et al. Tmbim1 is a multivesicular body regulator that protects against non-alcoholic fatty liver disease in mice and monkeys by targeting the lysosomal degradation of Tlr4. Nat. Med. 23, 742–752 (2017).
Wang, P.X. et al. Interferon regulatory factor 9 is a key mediator of hepatic ischemia/reperfusion injury. J. Hepatol. 62, 111–120 (2015).
Ji, Y.X. et al. The ubiquitin E3 ligase TRAF6 exacerbates pathological cardiac hypertrophy via TAK1-dependent signalling. Nat. Commun. 7, 11267 (2016).
Brunt, E.M., Kleiner, D.E., Wilson, L.A., Belt, P. & Neuschwander-Tetri, B.A. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology 53, 810–820 (2011).
Sun, P. et al. Mindin deficiency protects the liver against ischemia/reperfusion injury. J. Hepatol. 63, 1198–1211 (2015).
Wang, P.X. et al. Hepatocyte TRAF3 promotes liver steatosis and systemic insulin resistance through targeting TAK1-dependent signalling. Nat. Commun. 7, 10592 (2016).
Gao, L. et al. Tumor necrosis factor receptor–associated factor 5 (Traf5) acts as an essential negative regulator of hepatic steatosis. J. Hepatol. 65, 125–136 (2016).
Acknowledgements
We thank H.-B. Shu (Medical Research Institute, Wuhan University) for providing ubiquitin and several of its derivative plasmids. We thank Y. Wang (University of California, Los Angeles) for his helpful suggestions regarding this research and for editing the manuscript. This work was supported by grants from the National Science Fund for Distinguished Young Scholars (no. 81425005, H.L.), the Key Project of the National Natural Science Foundation (no. 81330005 and no. 81630011, H.L.), the National Science and Technology Support Project (no. 2014BAI02B01 and no. 2015BAI08B01, H.L.), the National Key Research and Development Program (no. 2013YQ030923-05, H.L.; no. 2016YFF0101500, Z.-G.S.), the National Natural Science Foundation of China (no. 81770053, Z.-G.S.) and the Key Collaborative Project of the National Natural Science Foundation (no. 91639304, H.L. and Z.-G.S.).
Author information
Authors and Affiliations
Contributions
P.Z., P.-X.W., L.-P.Z. and X.Z. designed and performed experiments, analyzed data and wrote the manuscript; Y.-X.J., X.-J.Z., C.F., Y.-X.L., X.Y. and M.-M.G. performed experiments, analyzed data and provided useful advice regarding the manuscript; Y.Z. performed histology experiments; J.G. constructed the genetically engineered mice used in this study; S.T. established mouse NASH models and performed mouse experiments; X.-Y.Z. performed western blot analysis; X.-L.M., F.L., Z.W. and Z.H. helped design the project and edited the manuscript; Z.-G.S. and H.L. designed experiments, wrote the manuscript and supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures & Tables
Supplementary Figures 1–11 & Supplementary Tables 1–3 (PDF 21087 kb)
Rights and permissions
About this article
Cite this article
Zhang, P., Wang, PX., Zhao, LP. et al. The deubiquitinating enzyme TNFAIP3 mediates inactivation of hepatic ASK1 and ameliorates nonalcoholic steatohepatitis. Nat Med 24, 84–94 (2018). https://doi.org/10.1038/nm.4453
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4453
This article is cited by
-
Caspase 6 promotes innate immune activation by functional crosstalk between RIPK1-IκBα axis in liver inflammation
Cell Communication and Signaling (2023)
-
Single-cell metabolic profiling reveals subgroups of primary human hepatocytes with heterogeneous responses to drug challenge
Genome Biology (2023)
-
Ablation of the deubiquitinase USP15 ameliorates nonalcoholic fatty liver disease and nonalcoholic steatohepatitis
Experimental & Molecular Medicine (2023)
-
Cullin-associated and neddylation-dissociated protein 1 (CAND1) alleviates NAFLD by reducing ubiquitinated degradation of ACAA2
Nature Communications (2023)
-
Functions of MAP3Ks in antiviral immunity
Immunologic Research (2023)