Abstract
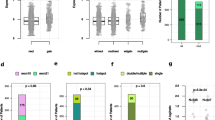
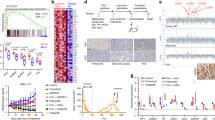
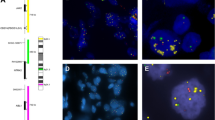
Tumor recurrence remains the main reason for breast cancer–associated mortality, and there are unmet clinical demands for the discovery of new biomarkers and development of treatment solutions to benefit patients with breast cancer at high risk of recurrence. Here we report the identification of chromosomal copy-number amplification at 1q21.3 that is enriched in subpopulations of breast cancer cells bearing characteristics of tumor-initiating cells (TICs) and that strongly associates with breast cancer recurrence. Amplification is present in ∼10–30% of primary tumors but in more than 70% of recurrent tumors, regardless of breast cancer subtype. Detection of amplification in cell-free DNA (cfDNA) from blood is strongly associated with early relapse in patients with breast cancer and could also be used to track the emergence of tumor resistance to chemotherapy. We further show that 1q21.3-encoded S100 calcium-binding protein (S100A) family members, mainly S100A7, S100A8, and S100A9 (S100A7/8/9), and IL-1 receptor–associated kinase 1 (IRAK1) establish a reciprocal feedback loop driving tumorsphere growth. Notably, this functional circuitry can be disrupted by the small-molecule kinase inhibitor pacritinib, leading to preferential impairment of the growth of 1q21.3-amplified breast tumors. Our study uncovers the 1q21.3-directed S100A7/8/9–IRAK1 feedback loop as a crucial component of breast cancer recurrence, serving as both a trackable biomarker and an actionable therapeutic target for breast cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Al-Hajj, M., Wicha, M.S., Benito-Hernandez, A., Morrison, S.J. & Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 100, 3983–3988 (2003).
Nguyen, L.V., Vanner, R., Dirks, P. & Eaves, C.J. Cancer stem cells: an evolving concept. Nat. Rev. Cancer 12, 133–143 (2012).
Mitra, A., Mishra, L. & Li, S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget 6, 10697–10711 (2015).
Ginestier, C. et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1, 555–567 (2007).
Marcato, P. et al. Aldehyde dehydrogenase 1A3 influences breast cancer progression via differential retinoic acid signaling. Mol. Oncol. 9, 17–31 (2015).
Marcato, P. et al. Aldehyde dehydrogenase activity of breast cancer stem cells is primarily due to isoform ALDH1A3 and its expression is predictive of metastasis. Stem Cells 29, 32–45 (2011).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Ciriello, G. et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell 163, 506–519 (2015).
Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, pl1 (2013).
Gyoőrffy, B., Surowiak, P., Budczies, J. & Lánczky, A. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS One 8, e82241 (2013).
Pereira, B. et al. The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat. Commun. 7, 11479 (2016).
Curtis, C. et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 486, 346–352 (2012).
Carreira, S. et al. Tumor clone dynamics in lethal prostate cancer. Sci. Transl. Med. 6, 254ra125 (2014).
Gevensleben, H. et al. Noninvasive detection of HER2 amplification with plasma DNA digital PCR. Clin. Cancer Res. 19, 3276–3284 (2013).
Miotke, L., Lau, B.T., Rumma, R.T. & Ji, H.P. High sensitivity detection and quantitation of DNA copy number and single nucleotide variants with single color droplet digital PCR. Anal. Chem. 86, 2618–2624 (2014).
Pearson, A. et al. High-level clonal FGFR amplification and response to FGFR inhibition in a translational clinical trial. Cancer Discov. 6, 838–851 (2016).
Romanel, A. et al. Plasma AR and abiraterone-resistant prostate cancer. Sci. Transl. Med. 7, 312re10 (2015).
Shoda, K. et al. Monitoring the HER2 copy number status in circulating tumor DNA by droplet digital PCR in patients with gastric cancer. Gastric Cancer 20, 126–135 (2016).
Alix-Panabières, C. & Pantel, K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 6, 479–491 (2016).
Chan, D. et al. Phase II study of gemcitabine and carboplatin in metastatic breast cancers with prior exposure to anthracyclines and taxanes. Invest. New Drugs 28, 859–865 (2010).
Wang, X. et al. S100A14, a mediator of epithelial–mesenchymal transition, regulates proliferation, migration and invasion of human cervical cancer cells. Am. J. Cancer Res. 5, 1484–1495 (2015).
Tanaka, M. et al. Co-expression of S100A14 and S100A16 correlates with a poor prognosis in human breast cancer and promotes cancer cell invasion. BMC Cancer 15, 53 (2015).
Bresnick, A.R., Weber, D.J. & Zimmer, D.B. S100 proteins in cancer. Nat. Rev. Cancer 15, 96–109 (2015).
Zhang, Q. et al. Downregulation of 425G>A variant of calcium-binding protein S100A14 associated with poor differentiation and prognosis in gastric cancer. J. Cancer Res. Clin. Oncol. 141, 691–703 (2015).
Cho, H. et al. The role of S100A14 in epithelial ovarian tumors. Oncotarget 5, 3482–3496 (2014).
Wee, Z.N. et al. IRAK1 is a therapeutic target that drives breast cancer metastasis and resistance to paclitaxel. Nat. Commun. 6, 8746 (2015).
Beauverd, Y., McLornan, D.P. & Harrison, C.N. Pacritinib: a new agent for the management of myelofibrosis?. Expert Opin. Pharmacother. 16, 2381–2390 (2015).
Singer, J.W. et al. Comprehensive kinase profile of pacritinib, a nonmyelosuppressive Janus kinase 2 inhibitor. J. Exp. Pharmacol. 16, 11–19 (2016).
Phase 1/2 study of pacritinib, a next generation JAK2/FLT3 inhibitor, in myelofibrosis or other myeloid malignancies. Hematol. Oncol. 9, 137 (2016).
Crowley, E., Di Nicolantonio, F., Loupakis, F. & Bardelli, A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 10, 472–484 (2013).
Siravegna, G. & Bardelli, A. Genotyping cell-free tumor DNA in the blood to detect residual disease and drug resistance. Genome Biol. 15, 449 (2014).
Dawson, S.J. et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N. Engl. J. Med. 368, 1199–1209 (2013).
Thress, K.S. et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non–small cell lung cancer harboring EGFR T790M. Nat. Med. 21, 560–562 (2015).
Siravegna, G. et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat. Med. 21, 795–801 (2015).
Garcia-Murillas, I. et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci. Transl. Med. 7, 302ra133 (2015).
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Marusyk, A., Almendro, V. & Polyak, K. Intra-tumour heterogeneity: a looking glass for cancer? Nat. Rev. Cancer 12, 323–334 (2012).
Yates, L.R. et al. Subclonal diversification of primary breast cancer revealed by multiregion sequencing. Nat. Med. 21, 751–759 (2015).
Forshew, T. et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci. Transl. Med. 4, 136ra68 (2012).
Murtaza, M. et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature 497, 108–112 (2013).
Beaver, J.A. et al. Detection of cancer DNA in plasma of patients with early-stage breast cancer. Clin. Cancer Res. 20, 2643–2650 (2014).
Duffy, M.J., Shering, S., Sherry, F., McDermott, E. & O'Higgins, N. CA 15-3: a prognostic marker in breast cancer. Int. J. Biol. Markers 15, 330–333 (2000).
Harris, L. et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J. Clin. Oncol. 25, 5287–5312 (2007).
Sturgeon, C. Practice guidelines for tumor marker use in the clinic. Clin. Chem. 48, 1151–1159 (2002).
Mermel, C.H. et al. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 12, R41 (2011).
Ono, S. et al. A direct plasma assay of circulating microRNA-210 of hypoxia can identify early systemic metastasis recurrence in melanoma patients. Oncotarget 6, 7053–7064 (2015).
McShane, L.M. et al. Reporting recommendations for tumor marker prognostic studies (REMARK). J. Natl. Cancer Inst. 97, 1180–1184 (2005).
Acknowledgements
This work was supported by the Agency for Science, Technology and Research of Singapore (A*STAR), the Margie Petersen Breast Cancer Program, and the Association of Breast Cancer (D.S.B.H.), Singapore Ministry of Health's National Medical Research Council (NMRC) Clinician Scientist Individual Research Grants (NMRC/CIRG/1464/2016 and NMRC/CG/017/2013 to E.Y.T.; NMRC/CSA/015/2009 and NMRC/CSA-SI/0004/2015 to S.C.L.), and the Danish Cancer Society (R99-A6362 and R146-A9164 to H.J.D.). This work was also supported by an A*STAR Graduate Academy (A*GA) SINGA (Singapore International Graduate Award) scholarship to G.O. We thank the Histopathology Department from the Institute of Molecular and Cell Biology, A*STAR, for their service in IHC staining and analysis.
Author information
Authors and Affiliations
Contributions
Q.Y. supervised the project and contributed to the design and interpretation of all experiments. J.Y.G. contributed to the design, conduct, and interpretation of all experiments. M.F. performed tumor sample preparation, genomic DNA and cfDNA extraction, RNA-seq, gene knockdown, and tumorsphere assays. Y.B. performed overexpression and tumorsphere assays. W.W. and P.L.L. performed western blot analyses. G.O. performed bioinformatics and statistical analyses. W.W., M.F., and S.M.J.M.Y. performed in vivo experiments. T.H.L. and A.S.T.L. performed DNA FISH analysis. P.W., A.R.K., M.B.L., and S.Y.L. contributed to collection of patient blood samples and clinical information. A.L. and S.S. contributed digital PCR analyses. W.L.T., Y.S.Y., D.S.B.H., H.J.D., S.C.L., and E.Y.T. provided crucial reagents and patient samples and contributed to clinical data analyses and interpretation. J.Y.G. and Q.Y. wrote the manuscript with input from all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 and Supplementary Tables 1–15. (PDF 1596 kb)
Supplementary Data
Uncropped Immunoblots. (PDF 754 kb)
Rights and permissions
About this article
Cite this article
Goh, J., Feng, M., Wang, W. et al. Chromosome 1q21.3 amplification is a trackable biomarker and actionable target for breast cancer recurrence. Nat Med 23, 1319–1330 (2017). https://doi.org/10.1038/nm.4405
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4405
This article is cited by
-
Intimate communications within the tumor microenvironment: stromal factors function as an orchestra
Journal of Biomedical Science (2023)
-
S100A8/A9 as a risk factor for breast cancer negatively regulated by DACH1
Biomarker Research (2023)
-
Exploring the prognostic value of S100A11 and its association with immune infiltration in breast cancer
Scientific Reports (2023)
-
Genomic profiling and pre-clinical modelling of breast cancer leptomeningeal metastasis reveals acquisition of a lobular-like phenotype
Nature Communications (2023)
-
S100A8 gene copy number and protein expression in breast cancer: associations with proliferation, histopathological grade and molecular subtypes
Breast Cancer Research and Treatment (2023)