Abstract
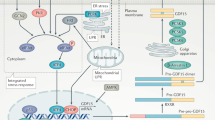
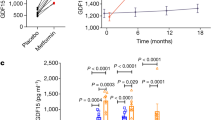
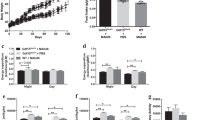
Growth differentiation factor 15 (GDF15), a distant member of the transforming growth factor (TGF)-β family, is a secreted protein that circulates as a 25-kDa dimer. In humans, elevated GDF15 correlates with weight loss, and the administration of GDF15 to mice with obesity reduces body weight, at least in part, by decreasing food intake. The mechanisms through which GDF15 reduces body weight remain poorly understood, because the cognate receptor for GDF15 is unknown. Here we show that recombinant GDF15 induces weight loss in mice fed a high-fat diet and in nonhuman primates with spontaneous obesity. Furthermore, we find that GDF15 binds with high affinity to GDNF family receptor α–like (GFRAL), a distant relative of receptors for a distinct class of the TGF-β superfamily ligands. Gfral is expressed in neurons of the area postrema and nucleus of the solitary tract in mice and humans, and genetic deletion of the receptor abrogates the ability of GDF15 to decrease food intake and body weight in mice. In addition, diet-induced obesity and insulin resistance are exacerbated in GFRAL-deficient mice, suggesting a homeostatic role for this receptor in metabolism. Finally, we demonstrate that GDF15-induced cell signaling requires the interaction of GFRAL with the coreceptor RET. Our data identify GFRAL as a new regulator of body weight and as the bona fide receptor mediating the metabolic effects of GDF15, enabling a more comprehensive assessment of GDF15 as a potential pharmacotherapy for the treatment of obesity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ng, M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781 (2014).
Heymsfield, S.B. & Wadden, T.A. Mechanisms, pathophysiology, and management of Obesity. N. Engl. J. Med. 376, 254–266 (2017).
Khera, R. et al. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. J. Am. Med. Assoc. 315, 2424–2434 (2016).
Johnen, H. et al. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat. Med. 13, 1333–1340 (2007).
Macia, L. et al. Macrophage inhibitory cytokine 1 (MIC-1/GDF15) decreases food intake, body weight and improves glucose tolerance in mice on normal & obesogenic diets. PLoS One 7, e34868 (2012).
Chrysovergis, K. et al. NAG-1/GDF-15 prevents obesity by increasing thermogenesis, lipolysis and oxidative metabolism. Int. J. Obes. 38, 1555–1564 (2014).
Wang, X. et al. hNAG-1 increases lifespan by regulating energy metabolism and insulin/IGF-1/mTOR signaling. Aging 6, 690–704 (2014).
Morrow, R.M. et al. Mitochondrial energy deficiency leads to hyperproliferation of skeletal muscle mitochondria and enhanced insulin sensitivity. Proc. Natl. Acad. Sci. USA 114, 2705–2710 (2017).
Chung, H.K. et al. Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. J. Cell Biol. 216, 149–165 (2017).
Tsai, V.W. et al. TGF-b superfamily cytokine MIC-1/GDF15 is a physiological appetite and body weight regulator. PLoS One 8, e55174 (2013).
Tsai, V.W. et al. The anorectic actions of the TGFβ cytokine MIC-1/GDF15 require an intact brainstem area postrema and nucleus of the solitary tract. PLoS One 9, e100370 (2014).
Brown, D.A. et al. Concentration in plasma of macrophage inhibitory cytokine-1 and risk of cardiovascular events in women: a nested case-control study. Lancet 359, 2159–2163 (2002).
Kempf, T. et al. Growth-differentiation factor-15 improves risk stratification in ST-segment elevation myocardial infarction. Eur. Heart J. 28, 2858–2865 (2007).
Kempf, T. et al. Growth differentiation factor 15 predicts future insulin resistance and impaired glucose control in obese nondiabetic individuals: results from the XENDOS trial. Eur. J. Endocrinol. 167, 671–678 (2012).
Tsai, V.W. et al. Serum levels of human MIC-1/GDF15 vary in a diurnal pattern, do not display a profile suggestive of a satiety factor and are related to BMI. PLoS One 10, e0133362 (2015).
Tsai, V.W. et al. Anorexia/cachexia of chronic diseases: a role for the TGF-β family cytokine MIC-1/GDF15. J. Cachexia Sarcopenia Muscle 3, 239–243 (2012).
Artz, A., Butz, S. & Vestweber, D. GDF-15 inhibits integrin activation and mouse neutrophil recruitment through the ALK-5/TGF-βRII heterodimer. Blood 128, 529–541 (2016).
Wang, C.Y., Huang, A.Q., Zhou, M.H. & Mei, Y.A. GDF15 regulates Kv2.1-mediated outward K+ current through the Akt/mTOR signalling pathway in rat cerebellar granule cells. Biochem. J. 460, 35–47 (2014).
de Jager, S.C. et al. Growth differentiation factor 15 deficiency protects against atherosclerosis by attenuating CCR2-mediated macrophage chemotaxis. J. Exp. Med. 208, 217–225 (2011).
Li, Z. et al. Identification, expression and functional characterization of the GRAL gene. J. Neurochem. 95, 361–376 (2005).
Hätinen, T., Holm, L. & Airaksinen, M.S. Loss of neurturin in frog--comparative genomics study of GDNF family ligand-receptor pairs. Mol. Cell. Neurosci. 34, 155–167 (2007).
Vieira, P., Thomas-Crusells, J. & Vieira, A. Internalization of glial cell-derived neurotrophic factor receptor GFR alpha 1 in the absence of the ret tyrosine kinase coreceptor. Cell. Mol. Neurobiol. 23, 43–55 (2003).
Hayes, M.R., Skibicka, K.P. & Grill, H.J. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology 149, 4059–4068 (2008).
Akhurst, R.J. & Hata, A. Targeting the TGFβ signalling pathway in disease. Nat. Rev. Drug Discov. 11, 790–811 (2012).
Allendorph, G.P., Isaacs, M.J., Kawakami, Y., Izpisua Belmonte, J.C. & Choe, S. BMP-3 and BMP-6 structures illuminate the nature of binding specificity with receptors. Biochemistry 46, 12238–12247 (2007).
Allendorph, G.P., Vale, W.W. & Choe, S. Structure of the ternary signaling complex of a TGF-beta superfamily member. Proc. Natl. Acad. Sci. USA 103, 7643–7648 (2006).
Greenwald, J. et al. The BMP7/ActRII extracellular domain complex provides new insights into the cooperative nature of receptor assembly. Mol. Cell 11, 605–617 (2003).
Parkash, V. & Goldman, A. Comparison of GFL-GFRalpha complexes: further evidence relating GFL bend angle to RET signalling. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 65, 551–558 (2009).
Goodman, K.M. et al. RET recognition of GDNF-GFRα1 ligand by a composite binding site promotes membrane-proximal self-association. Cell Rep. 8, 1894–1904 (2014).
Airaksinen, M.S. & Saarma, M. The GDNF family: signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 3, 383–394 (2002).
Alao, J.P., Michlikova, S., Dinér, P., Grøtli, M. & Sunnerhagen, P. Selective inhibition of RET mediated cell proliferation in vitro by the kinase inhibitor SPP86. BMC Cancer 14, 853 (2014).
Ding, Q. et al. Identification of macrophage inhibitory cytokine-1 in adipose tissue and its secretion as an adipokine by human adipocytes. Endocrinology 150, 1688–1696 (2009).
Verdeguer, F. et al. Brown adipose YY1 deficiency activates expression of secreted proteins linked to energy expenditure and prevents diet-induced obesity. Mol. Cell. Biol. 36, 184–196 (2015).
Hasebe, T. et al. Bone morphogenetic protein-binding endothelial regulator of liver sinusoidal endothelial cells induces iron overload in a fatty liver mouse model. J. Gastroenterol. 52, 341–351 (2017).
Quartu, M. et al. Tissue distribution of Ret, GFRalpha-1, GFRalpha-2 and GFRalpha-3 receptors in the human brainstem at fetal, neonatal and adult age. Brain Res. 1173, 36–52 (2007).
Bauskin, A.R. et al. The propeptide of macrophage inhibitory cytokine (MIC-1), a TGF-beta superfamily member, acts as a quality control determinant for correctly folded MIC-1. EMBO J. 19, 2212–2220 (2000).
Merchant, A.M. et al. An efficient route to human bispecific IgG. Nat. Biotechnol. 16, 677–681 (1998).
Turner, L. et al. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 498, 502–505 (2013).
Acknowledgements
We thank the following people for technical assistance in carrying out this work: B. Geist, Y. Wang, L. Lu, M. Husovsky (all at Janssen Research and Development). We thank E. Chi, S. Fenton, V. Stojanovic-Susulic, R. Swanson, J. Leonard and M. Erion (Janssen Research and Development) for support and insightful discussions. S.C.B., T.D.C.-M., C.R.C., and V.J.S. were employees of Kelly Outsourcing & Consulting Group. The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the US National Institutes of Health, and by the NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS.
Author information
Authors and Affiliations
Contributions
J.L.F., S.N., C.H., C.-N.C., M.J.H. and X.L.-S. contributed to protein design, generation and characterization. A.A.A. contributed to data interpretation and performed homology modeling. T.Q.D. designed and executed SPR experiments. J.A.C., S.C.B., V.J.S., X.L-S., C.-N.C. designed and executed in vitro studies. S.E.M. and S.M.R. designed all animal experiments which were executed by S.E.M., T.D.C.-M., and C.R.C. S.E.M., X.L.-S., C.-N.C., J.A.C. and S.M.R. contributed to all study design, data interpretation and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
All the authors are currently employed by Janssen Research and Development, as full-time employees or as contractors via Kelly Outsourcing & Consulting Group. There are patent documents related to this work.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13 and Supplementary Tables 1–3. (PDF 2267 kb)
Rights and permissions
About this article
Cite this article
Mullican, S., Lin-Schmidt, X., Chin, CN. et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med 23, 1150–1157 (2017). https://doi.org/10.1038/nm.4392
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4392
This article is cited by
-
Artesunate treats obesity in male mice and non-human primates through GDF15/GFRAL signalling axis
Nature Communications (2024)
-
Obesity and the kidney: mechanistic links and therapeutic advances
Nature Reviews Endocrinology (2024)
-
GDF15 as a key disease target and biomarker: linking chronic lung diseases and ageing
Molecular and Cellular Biochemistry (2024)
-
GDF15 induces chemoresistance to oxaliplatin by forming a reciprocal feedback loop with Nrf2 to maintain redox homeostasis in colorectal cancer
Cellular Oncology (2024)
-
Immunization with a multi-antigen targeted DNA vaccine eliminates chemoresistant pancreatic cancer by disrupting tumor-stromal cell crosstalk
Journal of Translational Medicine (2023)