Abstract
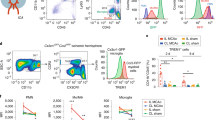
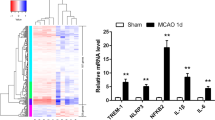
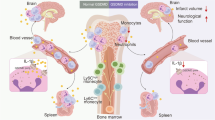
Damage-associated molecular patterns (DAMPs) trigger sterile inflammation after tissue injury, but the mechanisms underlying the resolution of inflammation remain unclear. In this study, we demonstrate that common DAMPs, such as high-mobility-group box 1 (HMGB1), peroxiredoxins (PRXs), and S100A8 and S100A9, were internalized through the class A scavenger receptors MSR1 and MARCO in vitro. In ischemic murine brain, DAMP internalization was largely mediated by MSR1. An elevation of MSR1 levels in infiltrating myeloid cells observed 3 d after experimental stroke was dependent on the transcription factor Mafb. Combined deficiency for Msr1 and Marco, or for Mafb alone, in infiltrating myeloid cells caused impaired clearance of DAMPs, more severe inflammation, and exacerbated neuronal injury in a murine model of ischemic stroke. The retinoic acid receptor (RAR) agonist Am80 increased the expression of Mafb, thereby enhancing MSR1 expression. Am80 exhibited therapeutic efficacy when administered, even at 24 h after the onset of experimental stroke. Our findings uncover cellular mechanisms contributing to DAMP clearance in resolution of the sterile inflammation triggered by tissue injury.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Moskowitz, M.A., Lo, E.H. & Iadecola, C. The science of stroke: mechanisms in search of treatments. Neuron 67, 181–198 (2010).
Lo, E.H. Degeneration and repair in central nervous system disease. Nat. Med. 16, 1205–1209 (2010).
Chamorro, Á., Dirnagl, U., Urra, X. & Planas, A.M. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 15, 869–881 (2016).
Iadecola, C. & Anrather, J. The immunology of stroke: from mechanisms to translation. Nat. Med. 17, 796–808 (2011).
Zimmer, S. et al. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci. Transl. Med. 8, 333ra50 (2016).
Dalli, J., Chiang, N. & Serhan, C.N. Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat. Med. 21, 1071–1075 (2015).
Buckley, C.D., Gilroy, D.W., Serhan, C.N., Stockinger, B. & Tak, P.P. The resolution of inflammation. Nat. Rev. Immunol. 13, 59–66 (2013).
Dirnagl, U. & Endres, M. Found in translation: preclinical stroke research predicts human pathophysiology, clinical phenotypes, and therapeutic outcomes. Stroke 45, 1510–1518 (2014).
Iadecola, C. & Anrather, J. Stroke research at a crossroad: asking the brain for directions. Nat. Neurosci. 14, 1363–1368 (2011).
Kono, H. & Rock, K.L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 8, 279–289 (2008).
Chen, G.Y. & Nuñez, G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826–837 (2010).
David, S. & Kroner, A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 12, 388–399 (2011).
Prinz, M. & Priller, J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat. Rev. Neurosci. 15, 300–312 (2014).
Hayakawa, K., Qiu, J. & Lo, E.H. Biphasic actions of HMGB1 signaling in inflammation and recovery after stroke. Ann. NY Acad. Sci. 1207, 50–57 (2010).
Qiu, J. et al. Early release of HMGB-1 from neurons after the onset of brain ischemia. J. Cereb. Blood Flow Metab. 28, 927–938 (2008).
Okuma, Y. et al. Anti–high mobility group box-1 antibody therapy for traumatic brain injury. Ann. Neurol. 72, 373–384 (2012).
Zhang, J. et al. Anti–high mobility group box-1 monoclonal antibody protects the blood–brain barrier from ischemia-induced disruption in rats. Stroke 42, 1420–1428 (2011).
Ziegler, G. et al. Mrp-8 and -14 mediate CNS injury in focal cerebral ischemia. Biochim. Biophys. Acta 1792, 1198–1204 (2009).
Vogl, T. et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 13, 1042–1049 (2007).
Loser, K. et al. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat. Med. 16, 713–717 (2010).
Klichko, V.I., Orr, W.C. & Radyuk, S.N. The role of peroxiredoxin 4 in inflammatory response and aging. Biochim. Biophys. Acta 1862, 265–273 (2016).
Salzano, S. et al. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc. Natl. Acad. Sci. USA 111, 12157–12162 (2014).
Riddell, J.R. et al. Peroxiredoxin 1 controls prostate cancer growth through Toll-like receptor 4–dependent regulation of tumor vasculature. Cancer Res. 71, 1637–1646 (2011).
Riddell, J.R., Wang, X.Y., Minderman, H. & Gollnick, S.O. Peroxiredoxin 1 stimulates secretion of proinflammatory cytokines by binding to TLR4. J. Immunol. 184, 1022–1030 (2010).
Shichita, T. et al. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat. Med. 18, 911–917 (2012).
Kuang, X. et al. Ligustilide ameliorates neuroinflammation and brain injury in focal cerebral ischemia/reperfusion rats: involvement of inhibition of TLR4/peroxiredoxin 6 signaling. Free Radic. Biol. Med. 71, 165–175 (2014).
Uzawa, A. et al. Increased serum peroxiredoxin 5 levels in myasthenia gravis. J. Neuroimmunol. 287, 16–18 (2015).
Rashidian, J. et al. Essential role of cytoplasmic cdk5 and Prx2 in multiple ischemic injury models, in vivo. J. Neurosci. 29, 12497–12505 (2009).
Dayon, L. et al. Brain extracellular fluid protein changes in acute stroke patients. J. Proteome Res. 10, 1043–1051 (2011).
Shichita, T. et al. Pivotal role of cerebral interleukin-17-producing γδ T cells in the delayed phase of ischemic brain injury. Nat. Med. 15, 946–950 (2009).
Ito, M. et al. Bruton's tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat. Commun. 6, 7360 (2015).
Benakis, C., Garcia-Bonilla, L., Iadecola, C. & Anrather, J. The role of microglia and myeloid immune cells in acute cerebral ischemia. Front. Cell. Neurosci. 8, 461 (2015).
Mildner, A. et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci. 10, 1544–1553 (2007).
Gliem, M. et al. Macrophages prevent hemorrhagic infarct transformation in murine stroke models. Ann. Neurol. 71, 743–752 (2012).
Schlegel, J., Neff, F. & Piontek, G. Serial induction of mutations by ethylnitrosourea in PC12 cells: a new model for a phenotypical characterization of the neurotoxic response to 6-hydroxydopamine. J. Neurosci. Methods 137, 215–220 (2004).
Canton, J., Neculai, D. & Grinstein, S. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol. 13, 621–634 (2013).
Lalancette-Hébert, M., Gowing, G., Simard, A., Weng, Y.C. & Kriz, J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J. Neurosci. 27, 2596–2605 (2007).
Li, S. et al. An age-related sprouting transcriptome provides molecular control of axonal sprouting after stroke. Nat. Neurosci. 13, 1496–1504 (2010).
Goldmann, T. et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci. 16, 1618–1626 (2013).
Wieghofer, P., Knobeloch, K.P. & Prinz, M. Genetic targeting of microglia. Glia 63, 1–22 (2015).
Aziz, A., Soucie, E., Sarrazin, S. & Sieweke, M.H. MafB/c-Maf deficiency enables self-renewal of differentiated functional macrophages. Science 326, 867–871 (2009).
Soucie, E.L. et al. Lineage-specific enhancers activate self-renewal genes in macrophages and embryonic stem cells. Science 351, aad5510 (2016).
Aziz, A. et al. Development of macrophages with altered actin organization in the absence of MafB. Mol. Cell. Biol. 26, 6808–6818 (2006).
Qian, B.Z. et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–225 (2011).
Hamada, M. et al. MafB promotes atherosclerosis by inhibiting foam-cell apoptosis. Nat. Commun. 5, 3147 (2014).
Matsushita, H. et al. A retinoic acid receptor agonist Am80 rescues neurons, attenuates inflammatory reactions, and improves behavioral recovery after intracerebral hemorrhage in mice. J. Cereb. Blood Flow Metab. 31, 222–234 (2011).
Katsuki, H. et al. Retinoic acid receptor stimulation protects midbrain dopaminergic neurons from inflammatory degeneration via BDNF-mediated signaling. J. Neurochem. 110, 707–718 (2009).
Klemann, C. et al. Synthetic retinoid AM80 inhibits Th17 cells and ameliorates experimental autoimmune encephalomyelitis. Am. J. Pathol. 174, 2234–2245 (2009).
Takeuchi, H. et al. Retinoid X receptor agonists modulate Foxp3+ regulatory T cell and Th17 cell differentiation with differential dependence on retinoic acid receptor activation. J. Immunol. 191, 3725–3733 (2013).
Desestret, V. et al. In vitro and in vivo models of cerebral ischemia show discrepancy in therapeutic effects of M2 macrophages. PLoS One 8, e67063 (2013).
Hu, X. et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 43, 3063–3070 (2012).
Lane, M.A. & Bailey, S.J. Role of retinoid signalling in the adult brain. Prog. Neurobiol. 75, 275–293 (2005).
Sarrazin, S. et al. MafB restricts M-CSF-dependent myeloid commitment divisions of hematopoietic stem cells. Cell 138, 300–313 (2009).
Suzuki, H. et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature 386, 292–296 (1997).
Chen, Y. et al. Defective microarchitecture of the spleen marginal zone and impaired response to a thymus-independent type 2 antigen in mice lacking scavenger receptors MARCO and SR-A. J. Immunol. 175, 8173–8180 (2005).
Muto, G. et al. TRAF6 is essential for maintenance of regulatory T cells that suppress Th2 type autoimmunity. PLoS One 8, e74639 (2013).
Yao, H. et al. Photothrombotic middle cerebral artery occlusion and reperfusion laser system in spontaneously hypertensive rats. Stroke 34, 2716–2721 (2003).
Bederson, J.B. et al. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 17, 472–476 (1986).
Balkaya, M., Kröber, J.M., Rex, A. & Endres, M. Assessing post-stroke behavior in mouse models of focal ischemia. J. Cereb. Blood Flow Metab. 33, 330–338 (2013).
Acknowledgements
We thank N. Shiino, A. Ino, and M. Asakawa for their technical assistance and H. Yao for his technical advice on the MCAO model. Msr1/Marco-deficient mice were kindly provided by K. Tryggvason (Duke–NUS Medical School). This work was supported by PRESTO from the Japan Science and Technology Agency (T.S.), a Grant-in-Aid for Scientific Research on Innovative Areas (Homeostatic regulation by various types of cell death) (15H01387) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) (T.S.), JSPS KAKENHI Grants-in-Aid for Young Scientists (B) (26870571) (T.S.) and (S) (25221305) (A.Y.), Advanced Research & Development Programs for Medical Innovation (AMED-CREST) (A.Y.), a Toray Science and Technology Grant (T.S.), the Takeda Science Foundation (T.S.), the Mochida Memorial Foundation for Medical and Pharmaceutical Research (T.S.), a Japan Heart Foundation Research Grant (T.S.), the SENSHIN Medical Research Foundation (A.Y.), Keio Gijuku Academic Developmental Funds (A.Y.), and Open Research for Young Academics and Specialists from MEXT (A.Y.).
Author information
Authors and Affiliations
Contributions
T.S. designed and performed the experiments, analyzed the data, and wrote the manuscript; Y.N., M.I., and K.K. performed the experiments; R.M. and H.O. provided technical advice; R.K. and S.T. provided Lysm-Cre; Mafbflox/flox mice; T.K. provided Msr1/Marco double-knockout mice; and A.Y. directed the entire study, designed the experiments, and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Tables 1–5 (PDF 5614 kb)
Rights and permissions
About this article
Cite this article
Shichita, T., Ito, M., Morita, R. et al. MAFB prevents excess inflammation after ischemic stroke by accelerating clearance of damage signals through MSR1. Nat Med 23, 723–732 (2017). https://doi.org/10.1038/nm.4312
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4312
This article is cited by
-
Promoting AMPK/SR-A1-mediated clearance of HMGB1 attenuates chemotherapy-induced peripheral neuropathy
Cell Communication and Signaling (2023)
-
MafB regulates NLRP3 inflammasome activation by sustaining p62 expression in macrophages
Communications Biology (2023)
-
Neuroimmune mechanisms and therapies mediating post-ischaemic brain injury and repair
Nature Reviews Neuroscience (2023)
-
Role of alarmins in poststroke inflammation and neuronal repair
Seminars in Immunopathology (2023)
-
Pulsed Electromagnetic Fields Protect Against Brain Ischemia by Modulating the Astrocytic Cholinergic Anti-inflammatory Pathway
Cellular and Molecular Neurobiology (2023)