Abstract
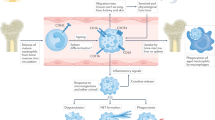
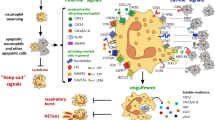
The production of neutrophil extracellular traps (NETs) is a process that enables neutrophils to help catch and kill bacteria. However, increasing evidence suggests that this process might also occur in noninfectious, sterile inflammation. In this Review, we describe the role of NETosis in autoimmunity, coagulation, acute injuries and cancer, and discuss NETs as potential therapeutic targets. Furthermore, we consider whether extracellular DNA is always detrimental in sterile inflammation and whether the source is always NETs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Belaaouaj, A. et al. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat. Med. 4, 615–618 (1998).
Takei, H., Araki, A., Watanabe, H., Ichinose, A. & Sendo, F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J. Leukoc. Biol. 59, 229–240 (1996).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Metzler, K.D., Goosmann, C., Lubojemska, A., Zychlinsky, A. & Papayannopoulos, V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 8, 883–896 (2014).
Papayannopoulos, V., Metzler, K.D., Hakkim, A. & Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 191, 677–691 (2010).
Yipp, B.G. & Kubes, P. NETosis: how vital is it? Blood 122, 2784–2794 (2013).
Clark, S.R. et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 13, 463–469 (2007).
Pilsczek, F.H. et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 185, 7413–7425 (2010).
Yipp, B.G. et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 18, 1386–1393 (2012).
Rochael, N.C. et al. Classical ROS-dependent and early/rapid ROS-independent release of neutrophil extracellular traps triggered by Leishmania parasites. Sci. Rep. 5, 18302 (2015).
Slaba, I. et al. Imaging the dynamic platelet-neutrophil response in sterile liver injury and repair in mice. Hepatology 62, 1593–1605 (2015).
Yousefi, S., Mihalache, C., Kozlowski, E., Schmid, I. & Simon, H.U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 16, 1438–1444 (2009).
Lood, C. et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 22, 146–153 (2016).
Kolaczkowska, E. et al. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat. Commun. 6, 6673 (2015).
Branzk, N. et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 15, 1017–1025 (2014).
Schauer, C. et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 20, 511–517 (2014).
Kolaczkowska, E. & Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013).
Fournier, B.M. & Parkos, C.A. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 5, 354–366 (2012).
Soehnlein, O. & Lindbom, L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 10, 427–439 (2010).
Hurst, S.M. et al. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity 14, 705–714 (2001).
Tsuda, Y. et al. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity 21, 215–226 (2004).
Zhang, D. et al. Neutrophil ageing is regulated by the microbiome. Nature 525, 528–532 (2015).
Gupta, S. & Kaplan, M.J. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat. Rev. Nephrol. 12, 402–413 (2016).
Denny, M.F. et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J. Immunol. 184, 3284–3297 (2010).
Garcia-Romo, G.S. et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra20 (2011).
Carmona-Rivera, C., Zhao, W., Yalavarthi, S. & Kaplan, M.J. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann. Rheum. Dis. 74, 1417–1424 (2015).
Villanueva, E. et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 187, 538–552 (2011).
Hakkim, A. et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. USA 107, 9813–9818 (2010).
Campbell, A.M., Kashgarian, M. & Shlomchik, M.J. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci. Transl. Med. 4, 157ra141 (2012).
Winkelstein, J.A. et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine 79, 155–169 (2000).
Sur Chowdhury, C. et al. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res. Ther. 16, R122 (2014).
Khandpur, R. et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 5, 178ra40 (2013).
Wang, W., Jian, Z., Guo, J. & Ning, X. Increased levels of serum myeloperoxidase in patients with active rheumatoid arthritis. Life Sci. 117, 19–23 (2014).
Christensen, A.D., Haase, C., Cook, A.D. & Hamilton, J.A. K/BxN serum-transfer arthritis as a model for human inflammatory arthritis. Front. Immunol. 7, 213 (2016).
Maicas, N. et al. Deficiency of Nrf2 accelerates the effector phase of arthritis and aggravates joint disease. Antioxid. Redox Signal. 15, 889–901 (2011).
Huang, X. et al. Neutrophils regulate humoral autoimmunity by restricting nterferon-γ production via the generation of reactive oxygen species. Cell Rep. 12, 1120–1132 (2015).
Lee, H.T. et al. The pathogenesis of systemic lupus erythematosus—From the viewpoint of oxidative stress and mitochondrial dysfunction. Mitochondrion 30, 1–7 (2016).
Rohrbach, A.S., Hemmers, S., Arandjelovic, S., Corr, M. & Mowen, K.A. PAD4 is not essential for disease in the K/BxN murine autoantibody-mediated model of arthritis. Arthritis Res. Ther. 14, R104 (2012).
Seri, Y. et al. Peptidylarginine deiminase type 4 deficiency reduced arthritis severity in a glucose-6-phosphate isomerase-induced arthritis model. Sci. Rep. 5, 13041 (2015).
Willis, V.C. et al. N-α-benzoyl-N5-(2-chloro-1-iminoethyl)-l-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J. Immunol. 186, 4396–4404 (2011).
Wang, Y. et al. Increased neutrophil elastase and proteinase 3 and augmented NETosis are closely associated with β-cell autoimmunity in patients with type 1 diabetes. Diabetes 63, 4239–4248 (2014).
Rodríguez-Espinosa, O., Rojas-Espinosa, O., Moreno-Altamirano, M.M., López-Villegas, E.O. & Sánchez-García, F.J. Metabolic requirements for neutrophil extracellular traps formation. Immunology 145, 213–224 (2015).
Wong, S.L. et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat. Med. 21, 815–819 (2015).
Joshi, M.B. et al. High glucose modulates IL-6 mediated immune homeostasis through impeding neutrophil extracellular trap formation. FEBS Lett. 587, 2241–2246 (2013).
Papayannopoulos, V. Sweet NETs, bitter wounds. Immunity 43, 223–225 (2015).
Fadini, G.P. et al. NETosis delays diabetic wound healing in mice and humans. Diabetes 65, 1061–1071 (2016).
Kallenberg, C.G. Key advances in the clinical approach to ANCA-associated vasculitis. Nat. Rev. Rheumatol. 10, 484–493 (2014).
Kessenbrock, K. et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 15, 623–625 (2009).
Pendergraft, W.F. III et al. Autoimmunity is triggered by cPR-3(105-201), a protein complementary to human autoantigen proteinase-3. Nat. Med. 10, 72–79 (2004).
Thieblemont, N., Wright, H.L., Edwards, S.W. & Witko-Sarsat, V. Human neutrophils in auto-immunity. Semin. Immunol. 28, 159–173 (2016).
Harper, L., Cockwell, P., Adu, D. & Savage, C.O. Neutrophil priming and apoptosis in anti-neutrophil cytoplasmic autoantibody-associated vasculitis. Kidney Int. 59, 1729–1738 (2001).
Söderberg, D. et al. Increased levels of neutrophil extracellular trap remnants in the circulation of patients with small vessel vasculitis, but an inverse correlation to anti-neutrophil cytoplasmic antibodies during remission. Rheumatology (Oxford) 54, 2085–2094 (2015).
Rao, A.N., Kazzaz, N.M. & Knight, J.S. Do neutrophil extracellular traps contribute to the heightened risk of thrombosis in inflammatory diseases? World J. Cardiol. 7, 829–842 (2015).
Imamoto, T. et al. Possible linkage between microscopic polyangiitis and thrombosis via neutrophil extracellular traps. Clin. Exp. Rheumatol. 32, 149–150 (2014).
Stakos, D.A. et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur. Heart J. 36, 1405–1414 (2015).
Kambas, K. et al. Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Ann. Rheum. Dis. 73, 1854–1863 (2014).
Semeraro, F. et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood 118, 1952–1961 (2011).
Massberg, S. et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 16, 887–896 (2010).
Martinod, K. et al. Neutrophil elastase-deficient mice form neutrophil extracellular traps in an experimental model of deep vein thrombosis. J. Thromb. Haemost. 14, 551–558 (2016).
Rangé, H. et al. Periodontal bacteria in human carotid atherothrombosis as a potential trigger for neutrophil activation. Atherosclerosis 236, 448–455 (2014).
Warnatsch, A., Ioannou, M., Wang, Q. & Papayannopoulos, V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 349, 316–320 (2015).
Swirski, F.K. & Nahrendorf, M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339, 161–166 (2013).
Nahrendorf, M. & Swirski, F.K. Immunology. Neutrophil-macrophage communication in inflammation and atherosclerosis. Science 349, 237–238 (2015).
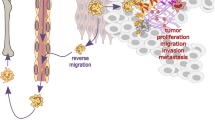
Demers, M. & Wagner, D.D. NETosis: a new factor in tumor progression and cancer-associated thrombosis. Semin. Thromb. Hemost. 40, 277–283 (2014).
Sionov, R.V., Fridlender, Z.G. & Granot, Z. The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron. 8, 125–158 (2015).
Jenne, C.N. & Kubes, P. Gastrointestinal cancer: Neutrophils and cancer: guilt by association. Nat. Rev. Gastroenterol. Hepatol. 13, 381–382 (2016).
Guglietta, S. et al. Coagulation induced by C3aR-dependent NETosis drives protumorigenic neutrophils during small intestinal tumorigenesis. Nat. Commun. 7, 11037 (2016).
Levi, M. Management of cancer-associated disseminated intravascular coagulation. Thromb. Res. 140 (Suppl. 1), S66–S70 (2016).
Ho-Tin-Noé, B. et al. Innate immune cells induce hemorrhage in tumors during thrombocytopenia. Am. J. Pathol. 175, 1699–1708 (2009).
Breitbach, C.J. et al. Targeting tumor vasculature with an oncolytic virus. Mol. Ther. 19, 886–894 (2011).
Cedervall, J. et al. Neutrophil extracellular traps accumulate in peripheral blood vessels and compromise organ function in tumor-bearing animals. Cancer Res. 75, 2653–2662 (2015).
Cools-Lartigue, J. et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Invest. 123, 3446–3458 (2013).
Hampson, P. et al. Neutrophil dysfunction, immature granulocytes, and cell-free DNA are early biomarkers of sepsis in burn-injured patients: a prospective observational cohort study. Ann. Surg. http://dx.doi.org/10.1097/SLA.0000000000001807 (2016).
Allam, R., Kumar, S.V., Darisipudi, M.N. & Anders, H.J. Extracellular histones in tissue injury and inflammation. J. Mol. Med. (Berl.) 92, 465–472 (2014).
Malachowa, N., Kobayashi, S.D., Freedman, B., Dorward, D.W. & DeLeo, F.R. Staphylococcus aureus leukotoxin GH promotes formation of neutrophil extracellular traps. J. Immunol. 191, 6022–6029 (2013).
Wang, J. & Kubes, P. A reservoir of mature cavity macrophages that can rapidly invade visceral organs to affect tissue repair. Cell 165, 668–678 (2016).
Garcia, R.J. et al. Attention deficit and hyperactivity disorder scores are elevated and respond to N-acetylcysteine treatment in patients with systemic lupus erythematosus. Arthritis Rheum. 65, 1313–1318 (2013).
Lai, Z.W. et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 64, 2937–2946 (2012).
Van Avondt, K., van der Linden, M., Naccache, P.H., Egan, D.A. & Meyaard, L. Signal inhibitory receptor on leukocytes-1 limits the formation of neutrophil extracellular traps, but preserves intracellular bacterial killing. J. Immunol. 196, 3686–3694 (2016).
Van Avondt, K., Fritsch-Stork, R., Derksen, R.H. & Meyaard, L. Ligation of signal inhibitory receptor on leukocytes-1 suppresses the release of neutrophil extracellular traps in systemic lupus erythematosus. PLoS One 8, e78459 (2013).
Martinod, K. et al. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood 125, 1948–1956 (2015).
Hemmers, S., Teijaro, J.R., Arandjelovic, S. & Mowen, K.A. PAD4-mediated neutrophil extracellular trap formation is not required for immunity against influenza infection. PLoS One 6, e22043 (2011).
Knight, J.S. et al. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann. Rheum. Dis. 74, 2199–2206 (2015).
Knight, J.S. et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ. Res. 114, 947–956 (2014).
Lewis, H.D. et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat. Chem. Biol. 11, 189–191 (2015).
Zheng, W. et al. PF-1355, a mechanism-based myeloperoxidase inhibitor, prevents immune complex vasculitis and anti-glomerular basement membrane glomerulonephritis. J. Pharmacol. Exp. Ther. 353, 288–298 (2015).
Sayah, D.M. et al. Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. Am. J. Respir. Crit. Care Med. 191, 455–463 (2015).
Macanovic, M. et al. The treatment of systemic lupus erythematosus (SLE) in NZB/W F1 hybrid mice; studies with recombinant murine DNase and with dexamethasone. Clin. Exp. Immunol. 106, 243–252 (1996).
Davis, J.C. Jr. et al. Recombinant human Dnase I (rhDNase) in patients with lupus nephritis. Lupus 8, 68–76 (1999).
Domingo-Gonzalez, R. et al. Inhibition of neutrophil extracellular trap formation after stem cell transplant by prostaglandin E2. Am. J. Respir. Crit. Care Med. 193, 186–197 (2016).
Shishikura, K. et al. Prostaglandin E2 inhibits neutrophil extracellular trap formation through production of cyclic AMP. Br. J. Pharmacol. 173, 319–331 (2016).
Barnado, A., Crofford, L.J. & Oates, J.C. At the Bedside: Neutrophil extracellular traps (NETs) as targets for biomarkers and therapies in autoimmune diseases. J. Leukoc. Biol. 99, 265–278 (2016).
Rai, R., Chauhan, S.K., Singh, V.V., Rai, M. & Rai, G. RNA-seq analysis reveals unique transcriptome signatures in systemic lupus erythematosus patients with distinct autoantibody specificities. PLoS One 11, e0166312 (2016).
Chang, H.H., Dwivedi, N., Nicholas, A.P. & Ho, I.C. The W620 polymorphism in PTPN22 disrupts its interaction with peptidylarginine deiminase type 4 and enhances citrullination and NETosis. Arthritis Rheumatol. 67, 2323–2334 (2015).
Acknowledgements
We thank C. Deppermann for proofreading the manuscript. Work in the authors' laboratory is supported by grants from the Canadian Institutes of Health Research, Alberta Innovates Health Solutions, the Heart and Stroke Foundation of Canada and the Canada Research Chairs program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Jorch, S., Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med 23, 279–287 (2017). https://doi.org/10.1038/nm.4294
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4294
This article is cited by
-
The scaffold of neutrophil extracellular traps promotes CCA progression and modulates angiogenesis via ITGAV/NFκB
Cell Communication and Signaling (2024)
-
Neutrophil extracellular traps mediated by platelet microvesicles promote thrombosis and brain injury in acute ischemic stroke
Cell Communication and Signaling (2024)
-
What is the actual relationship between neutrophil extracellular traps and COVID-19 severity? A longitudinal study
Respiratory Research (2024)
-
Neutrophil-mediated type IV collagen degradation is elevated in patients with mild endoscopic ulcerative colitis reflecting early mucosal destruction
Scientific Reports (2024)
-
PRL2 regulates neutrophil extracellular trap formation which contributes to severe malaria and acute lung injury
Nature Communications (2024)