Abstract
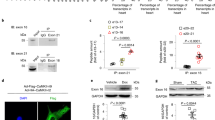
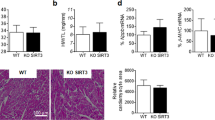
The voltage-gated cardiac Na+ channel (Nav1.5), encoded by the SCN5A gene, conducts the inward depolarizing cardiac Na+ current (INa) and is vital for normal cardiac electrical activity. Inherited loss-of-function mutations in SCN5A lead to defects in the generation and conduction of the cardiac electrical impulse and are associated with various arrhythmia phenotypes1. Here we show that sirtuin 1 deacetylase (Sirt1) deacetylates Nav1.5 at lysine 1479 (K1479) and stimulates INa via lysine-deacetylation-mediated trafficking of Nav1.5 to the plasma membrane. Cardiac Sirt1 deficiency in mice induces hyperacetylation of K1479 in Nav1.5, decreases expression of Nav1.5 on the cardiomyocyte membrane, reduces INa and leads to cardiac conduction abnormalities and premature death owing to arrhythmia. The arrhythmic phenotype of cardiac-Sirt1-deficient mice recapitulated human cardiac arrhythmias resulting from loss of function of Nav1.5. Increased Sirt1 activity or expression results in decreased lysine acetylation of Nav1.5, which promotes the trafficking of Nav1.5 to the plasma membrane and stimulation of INa. As compared to wild-type Nav1.5, Nav1.5 with K1479 mutated to a nonacetylatable residue increases peak INa and is not regulated by Sirt1, whereas Nav1.5 with K1479 mutated to mimic acetylation decreases INa. Nav1.5 is hyperacetylated on K1479 in the hearts of patients with cardiomyopathy and clinical conduction disease. Thus, Sirt1, by deacetylating Nav1.5, plays an essential part in the regulation of INa and cardiac electrical activity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ruan, Y., Liu, N. & Priori, S.G. Sodium channel mutations and arrhythmias. Nat. Rev. Cardiol. 6, 337–348 (2009).
Chen-Izu, Y. et al. Na+ channel function, regulation, structure, trafficking and sequestration. J. Physiol. (Lond.) 593, 1347–1360 (2015).
The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N. Engl. J. Med. 321, 406–412 (1989).
Marionneau, C. & Abriel, H. Regulation of the cardiac Na+ channel NaV1.5 by post-translational modifications. J. Mol. Cell. Cardiol. 82, 36–47 (2015).
Pei, Z., Xiao, Y., Meng, J., Hudmon, A. & Cummins, T.R. Cardiac sodium channel palmitoylation regulates channel availability and myocyte excitability with implications for arrhythmia generation. Nat. Commun. 7, 12035 (2016).
Liu, M. et al. Cardiac Na+ current regulation by pyridine nucleotides. Circ. Res. 105, 737–745 (2009).
Valdivia, C.R., Ueda, K., Ackerman, M.J. & Makielski, J.C. GPD1L links redox state to cardiac excitability by PKC-dependent phosphorylation of the sodium channel SCN5A. Am. J. Physiol. Heart Circ. Physiol. 297, H1446–H1452 (2009).
Grant, A.O. et al. Long QT syndrome, Brugada syndrome, and conduction system disease are linked to a single sodium channel mutation. J. Clin. Invest. 110, 1201–1209 (2002).
Zhang, Z.S., Tranquillo, J., Neplioueva, V., Bursac, N. & Grant, A.O. Sodium channel kinetic changes that produce Brugada syndrome or progressive cardiac conduction system disease. Am. J. Physiol. Heart Circ. Physiol. 292, H399–H407 (2007).
Hallaq, H. et al. Quantitation of protein kinase A-mediated trafficking of cardiac sodium channels in living cells. Cardiovasc. Res. 72, 250–261 (2006).
Hallaq, H. et al. Activation of protein kinase C alters the intracellular distribution and mobility of cardiac Na+ channels. Am. J. Physiol. Heart Circ. Physiol. 302, H782–H789 (2012).
Royer, A. et al. Mouse model of SCN5A-linked hereditary Lenègre's disease: age-related conduction slowing and myocardial fibrosis. Circulation 111, 1738–1746 (2005).
van Bemmelen, M.X. et al. Cardiac voltage-gated sodium channel Nav1.5 is regulated by Nedd4-2 mediated ubiquitination. Circ. Res. 95, 284–291 (2004).
Bosch-Presegué, L. et al. Stabilization of Suv39H1 by SirT1 is part of oxidative stress response and ensures genome protection. Mol. Cell 42, 210–223 (2011).
Benson, D.W. et al. Congenital sick sinus syndrome caused by recessive mutations in the cardiac sodium channel gene (SCN5A). J. Clin. Invest. 112, 1019–1028 (2003).
Schott, J.J. et al. Cardiac conduction defects associate with mutations in SCN5A. Nat. Genet. 23, 20–21 (1999).
Tan, H.L. et al. A sodium-channel mutation causes isolated cardiac conduction disease. Nature 409, 1043–1047 (2001).
Lei, M., Zhang, H., Grace, A.A. & Huang, C.L. SCN5A and sinoatrial node pacemaker function. Cardiovasc. Res. 74, 356–365 (2007).
Papadatos, G.A. et al. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. Proc. Natl. Acad. Sci. USA 99, 6210–6215 (2002).
Probst, V. et al. Haploinsufficiency in combination with aging causes SCN5A-linked hereditary Lenègre disease. J. Am. Coll. Cardiol. 41, 643–652 (2003).
Yokokawa, M. et al. Comparison of long-term follow-up of electrocardiographic features in Brugada syndrome between the SCN5A-positive probands and the SCN5A-negative probands. Am. J. Cardiol. 100, 649–655 (2007).
de Jong, S., van Veen, T.A., van Rijen, H.V. & de Bakker, J.M. Fibrosis and cardiac arrhythmias. J. Cardiovasc. Pharmacol. 57, 630–638 (2011).
Wang, Q. et al. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell 80, 805–811 (1995).
Chandra, R., Starmer, C.F. & Grant, A.O. Multiple effects of KPQ deletion mutation on gating of human cardiac Na+ channels expressed in mammalian cells. Am. J. Physiol. 274, H1643–H1654 (1998).
Schulze-Bahr, E. et al. Sodium channel gene (SCN5A) mutations in 44 index patients with Brugada syndrome: different incidences in familial and sporadic disease. Hum. Mutat. 21, 651–652 (2003).
Gao, G. et al. Role of RBM25/LUC7L3 in abnormal cardiac sodium channel splicing regulation in human heart failure. Circulation 124, 1124–1131 (2011).
Herren, A.W., Bers, D.M. & Grandi, E. Post-translational modifications of the cardiac Na channel: contribution of CaMKII-dependent phosphorylation to acquired arrhythmias. Am. J. Physiol. Heart Circ. Physiol. 305, H431–H445 (2013).
Valdivia, C.R. et al. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J. Mol. Cell. Cardiol. 38, 475–483 (2005).
McNair, W.P. et al. SCN5A mutations associate with arrhythmic dilated cardiomyopathy and commonly localize to the voltage-sensing mechanism. J. Am. Coll. Cardiol. 57, 2160–2168 (2011).
Zhao, Y. et al. Acetylation of p53 at lysine 373/382 by the histone deacetylase inhibitor depsipeptide induces expression of p21(Waf1/Cip1). Mol. Cell. Biol. 26, 2782–2790 (2006).
Yoon, J.Y. et al. A novel Na+ channel agonist, dimethyl lithospermate B, slows Na+ current inactivation and increases action potential duration in isolated rat ventricular myocytes. Br. J. Pharmacol. 143, 765–773 (2004).
Mattagajasingh, I. et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 104, 14855–14860 (2007).
Mitchell, G.F., Jeron, A. & Koren, G. Measurement of heart rate and Q-T interval in the conscious mouse. Am. J. Physiol. 274, H747–H751 (1998).
Petric, S. et al. In vivo electrophysiological characterization of TASK-1 deficient mice. Cell. Physiol. Biochem. 30, 523–537 (2012).
Burgoyne, J.R. et al. Oxidation of HRas cysteine thiols by metabolic stress prevents palmitoylation in vivo and contributes to endothelial cell apoptosis. FASEB J. 26, 832–841 (2012).
Dickey, D.M., Dries, D.L., Margulies, K.B. & Potter, L.R. Guanylyl cyclase (GC)-A and GC-B activities in ventricles and cardiomyocytes from failed and non-failed human hearts: GC-A is inactive in the failed cardiomyocyte. J. Mol. Cell. Cardiol. 52, 727–732 (2012).
Acknowledgements
This work was supported by NIH grant HL115955 to K.I. and B.L., and University of Iowa Endowed Professorship to K.I. M.K. was supported by a Cardiovascular Institutional Research Fellowship (T32 HL007121, PI: F. Abboud). Q.L. was supported by the Training Program in Hematology: Molecular & Cell Biology of Blood Cells (T32 HL007344, PI: S. Lentz). Procurement of human heart tissue was enabled by NIH grant HL105993 to K.B.M. Proteomics studies were supported by NIH grants HL068758 and HL104017 (PI: R.A. Cohen) and DK103750 to M.M.B. We acknowledge NIH support for services that we received under contract HHSN268201000031 (PI: C. Costello). We acknowledge J. Lee at the Dana-Farber Molecular Biology Core Facility for his support with the MALDI and Orbitrap MS instruments. Cardiomyocyte imaging data were acquired at the University of Iowa Central Microscopy Research Facility, with NIH 1S10RR02543901 funding for the shared Zeiss LSM 710 Confocal Microscope. Mouse echocardiograms were made possible with the NIH shared instrument grant 1S10ODO019941. We thank S. Dudley for the Nav1.5-expressing cell line, T. Kouzarides for the Sirt1 constructs and D. Roden for the Scn5a+/− mice.
Author information
Authors and Affiliations
Contributions
K.I. and B.L. designed and conceived the project. A.V., C.M.L., J.-Y.Y., A.N., S.K., G.M.M., J.S.J., Q.L., Y.-R.K., M.K., J.L., M.G., A.K., H.M., X. Zhu, X.G., W.K., X. Zhang, S.D., S.-B.J., V.K. and M.M.B. carried out experimental work, analyzed data and participated in data interpretation. R.L.B. and D.S.M. helped with data interpretation. K.B.M. provided critical human heart tissue and data set. K.I., B.L. and A.V. wrote the manuscript. K.I. and B.L. supervised the research and interpreted the data.
Corresponding authors
Ethics declarations
Competing interests
K.I. and B.L. have submitted a provisional patent filing on the use of Sirt1 activators for treatment of arrhythmias. The other authors declare that they have no competing interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 1–8 (PDF 1469 kb)
Rights and permissions
About this article
Cite this article
Vikram, A., Lewarchik, C., Yoon, JY. et al. Sirtuin 1 regulates cardiac electrical activity by deacetylating the cardiac sodium channel. Nat Med 23, 361–367 (2017). https://doi.org/10.1038/nm.4284
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4284
This article is cited by
-
Dapagliflozin attenuates pressure overload-induced myocardial remodeling in mice via activating SIRT1 and inhibiting endoplasmic reticulum stress
Acta Pharmacologica Sinica (2022)
-
Harnessing NAD+ Metabolism as Therapy for Cardiometabolic Diseases
Current Heart Failure Reports (2022)
-
The mitochondrial calcium uniporter promotes arrhythmias caused by high-fat diet
Scientific Reports (2021)
-
Deacetylation as a receptor-regulated direct activation switch for pannexin channels
Nature Communications (2021)
-
Sirtuins and the circadian clock interplay in cardioprotection: focus on sirtuin 1
Cellular and Molecular Life Sciences (2021)