Abstract
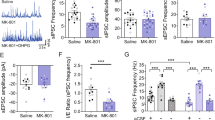
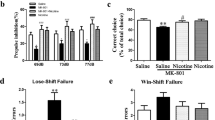
The prefrontal cortex (PFC) underlies higher cognitive processes1 that are modulated by nicotinic acetylcholine receptor (nAChR) activation by cholinergic inputs2. PFC spontaneous default activity3 is altered in neuropsychiatric disorders4, including schizophrenia5—a disorder that can be accompanied by heavy smoking6. Recently, genome-wide association studies (GWAS) identified single-nucleotide polymorphisms (SNPs) in the human CHRNA5 gene, encoding the α5 nAChR subunit, that increase the risks for both smoking and schizophrenia7,8. Mice with altered nAChR gene function exhibit PFC-dependent behavioral deficits9,10,11, but it is unknown how the corresponding human polymorphisms alter the cellular and circuit mechanisms underlying behavior. Here we show that mice expressing a human α5 SNP exhibit neurocognitive behavioral deficits in social interaction and sensorimotor gating tasks. Two-photon calcium imaging in awake mouse models showed that nicotine can differentially influence PFC pyramidal cell activity by nAChR modulation of layer II/III hierarchical inhibitory circuits. In α5-SNP-expressing and α5-knockout mice, lower activity of vasoactive intestinal polypeptide (VIP) interneurons resulted in an increased somatostatin (SOM) interneuron inhibitory drive over layer II/III pyramidal neurons. The decreased activity observed in α5-SNP-expressing mice resembles the hypofrontality observed in patients with psychiatric disorders, including schizophrenia and addiction5,12. Chronic nicotine administration reversed this hypofrontality, suggesting that administration of nicotine may represent a therapeutic strategy for the treatment of schizophrenia, and a physiological basis for the tendency of patients with schizophrenia to self-medicate by smoking13.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dehaene, S. & Changeux, J.-P. Experimental and theoretical approaches to conscious processing. Neuron 70, 200–227 (2011).
Bloem, B., Poorthuis, R.B. & Mansvelder, H.D. Cholinergic modulation of the medial prefrontal cortex: the role of nicotinic receptors in attention and regulation of neuronal activity. Front. Neural Circuits 8, 17 (2014).
Raichle, M.E. The brain's default mode network. Annu. Rev. Neurosci. 38, 433–447 (2015).
Buckner, R.L., Andrews-Hanna, J.R. & Schacter, D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. NY Acad. Sci. 1124, 1–38 (2008).
Barch, D.M. et al. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch. Gen. Psychiatry 58, 280–288 (2001).
de Leon, J. & Diaz, F.J. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr. Res. 76, 135–157 (2005).
Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat. Genet. 42, 441–447 (2010).
Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427 (2014).
Young, J.W. et al. Impaired attention is central to the cognitive deficits observed in α7 deficient mice. Eur. Neuropsychopharmacol. 17, 145–155 (2007).
Guillem, K. et al. Nicotinic acetylcholine receptor β2 subunits in the medial prefrontal cortex control attention. Science 333, 888–891 (2011).
Bailey, C.D.C., De Biasi, M., Fletcher, P.J. & Lambe, E.K. The nicotinic acetylcholine receptor α5 subunit plays a key role in attention circuitry and accuracy. J. Neurosci. 30, 9241–9252 (2010).
Hong, L.E. et al. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc. Natl. Acad. Sci. USA 107, 13509–13514 (2010).
Adler, L.E. et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr. Bull. 24, 189–202 (1998).
Robbins, T.W. & Roberts, A.C. Differential regulation of fronto-executive function by the monoamines and acetylcholine. Cereb. Cortex 17 (Suppl. 1), i151–i160 (2007).
Poorthuis, R.B. et al. Layer-specific modulation of the prefrontal cortex by nicotinic acetylcholine receptors. Cereb. Cortex 23, 148–161 (2013).
Sarter, M., Parikh, V. & Howe, W.M. Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nat. Rev. Neurosci. 10, 383–390 (2009).
Sinkus, M.L. et al. The human CHRNA7 and CHRFAM7A genes: a review of the genetics, regulation, and function. Neuropharmacology 96 (Part B), 274–288 (2015).
Bierut, L.J. et al. Variants in nicotinic receptors and risk for nicotine dependence. Am. J. Psychiatry 165, 1163–1171 (2008).
Sciaccaluga, M. et al. Crucial role of nicotinic α5 subunit variants for Ca2+ fluxes in ventral midbrain neurons. FASEB J. 29, 3389–3398 (2015).
Proulx, E., Piva, M., Tian, M.K., Bailey, C.D.C. & Lambe, E.K. Nicotinic acetylcholine receptors in attention circuitry: the role of layer VI neurons of prefrontal cortex. Cell. Mol. Life Sci. 71, 1225–1244 (2014).
Derntl, B. & Habel, U. Deficits in social cognition: a marker for psychiatric disorders? Eur. Arch. Psychiatry Clin. Neurosci. 261 (Suppl. 2), S145–S149 (2011).
Nadler, J.J. et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 3, 303–314 (2004).
Braff, D.L., Geyer, M.A. & Swerdlow, N.R. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl.) 156, 234–258 (2001).
Porter, J.T. et al. Selective excitation of subtypes of neocortical interneurons by nicotinic receptors. J. Neurosci. 19, 5228–5235 (1999).
Pi, H.-J. et al. Cortical interneurons that specialize in disinhibitory control. Nature 503, 521–524 (2013).
Poorthuis, R.B., Bloem, B., Verhoog, M.B. & Mansvelder, H.D. Layer-specific interference with cholinergic signaling in the prefrontal cortex by smoking concentrations of nicotine. J. Neurosci. 33, 4843–4853 (2013).
Kawaguchi, Y. & Kubota, Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb. Cortex 7, 476–486 (1997).
McGehee, D.S. & Role, L.W. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu. Rev. Physiol. 57, 521–546 (1995).
Sander, J.D. & Joung, J.K. CRISPR–Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 32, 347–355 (2014).
Pfeffer, C.K., Xue, M., He, M., Huang, Z.J. & Scanziani, M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat. Neurosci. 16, 1068–1076 (2013).
Levin, E.D., McClernon, F.J. & Rezvani, A.H. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl.) 184, 523–539 (2006).
Hambsch, B. et al. Chronic nicotine improves short-term memory selectively in a G72 mouse model of schizophrenia. Br. J. Pharmacol. 171, 1758–1771 (2014).
Matta, S.G. et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl.) 190, 269–319 (2007).
Morel, C. et al. Nicotine consumption is regulated by a human polymorphism in dopamine neurons. Mol. Psychiatry 19, 930–936 (2014).
Orr-Urtreger, A. et al. Mice deficient in the α7 neuronal nicotinic acetylcholine receptor lack α-bungarotoxin binding sites and hippocampal fast nicotinic currents. J. Neurosci. 17, 9165–9171 (1997).
Picciotto, M.R. et al. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature 374, 65–67 (1995).
Salas, R. et al. The nicotinic acetylcholine receptor subunit α5 mediates short-term effects of nicotine in vivo. Mol. Pharmacol. 63, 1059–1066 (2003).
Fuchs, E.C. et al. Genetically altered AMPA-type glutamate receptor kinetics in interneurons disrupt long-range synchrony of gamma oscillation. Proc. Natl. Acad. Sci. USA 98, 3571–3576 (2001).
Tolu, S. et al. A versatile system for the neuronal subtype specific expression of lentiviral vectors. FASEB J. 24, 723–730 (2010).
Arroyo, S., Bennett, C., Aziz, D., Brown, S.P. & Hestrin, S. Prolonged disynaptic inhibition in the cortex mediated by slow, non-α7 nicotinic excitation of a specific subset of cortical interneurons. J. Neurosci. 32, 3859–3864 (2012).
Prönneke, A. et al. Characterizing VIP neurons in the barrel cortex of VIPcre/tdTomato mice reveals layer-specific differences. Cereb. Cortex 25, 4854–4868 (2015).
Choi, G.B. et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939 (2016).
Holtmaat, A. et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 4, 1128–1144 (2009).
Naldini, L. et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272, 263–267 (1996).
Maskos, U. et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436, 103–107 (2005).
Lepousez, G. & Lledo, P.-M. Odor discrimination requires proper olfactory fast oscillations in awake mice. Neuron 80, 1010–1024 (2013).
Guo, Z.V. et al. Procedures for behavioral experiments in head-fixed mice. PLoS One 9, e88678 (2014).
Besson, M. et al. Long-term effects of chronic nicotine exposure on brain nicotinic receptors. Proc. Natl. Acad. Sci. USA 104, 8155–8160 (2007).
Acknowledgements
We would like to thank S. Pons for valuable support and discussions on lentiviral constructs, and M. Soudant for production of lentiviral vectors. We also acknowledge the Pasteur Institute Shared Neuroscience Department imaging facility funded by Ile-de-France Domaine d'Intérêt Majeur (DIM/NeRF). We acknowledge the GENIE Program and the Janelia Research Campus and specifically V. Jayaraman, D.S. Kim, L.L. Looger and K. Svoboda from the GENIE Project, Janelia Research Campus, Howard Hughes Medical Institute for making AAV.Syn.GCaMP6f and AAV.Syn.Flex.GCaMP6f available. Finally, we thank G. Fond and M. Groszer for comments on the manuscript. F.K. is a scholar of the Pasteur Paris University Doctoral Program (PPU) and received a stipend from the Stavros Niarchos Foundation. This work was supported by the CNRS UMR 3571, the Fondation de la Recherche Médicale (FRM grant DPA20140629803), the Agence Nationale de la Recherche (ANR), the Laboratoire d'Excellence BIO-PSY (including salary support to F.K., AAP Fin de Thèse 2015), the program PasteurInnov 2012, the FP7 ERANET program NICO-GENE, Grant Agreement n009 BLANC 20092009BLANC 20, the European Commission FP7 RTD Project HEALTH-2009-Neurocyp.08-202088 Grant 242167, French National Cancer Institute Grant CANCEROPOLE IDF 2016-1-TABAC-01-IP-1 MASKOS (all to U.M.), and NIH grants CA089392 and DA015663 (to J.S.). The laboratories of U.M., B.S.G. and D.A.D. are part of the École des Neurosciences de Paris Ile-de-France RTRA network. U.M. and D.A.D. are members of the Laboratory of Excellence, LabEx BIO-PSY. As such, this work was supported by French state funds managed by the ANR within the Investissements d'Avenir program under reference ANR-11-IDEX-0004-02. B.S.G. is a member of the Laboratory of Excellence, LabEx IEC. B.S.G. acknowledges support from the Russian Academic Excellence Project '5-100'.
Author information
Authors and Affiliations
Contributions
F.K. and U.M. conceived and designed research. F.K. performed experiments. F.K., K.A.S. and D.A.D. established the imaging technique. F.K., M.R., D.T. and D.A.D. designed the analysis code. F.K. and M.R. analyzed data. H.C.O'N., J.L. and C.A.H. performed behavioral experiments. M.W. contributed the VIP-Cre/tdTomato mice. J.A.S. developed the α5SNP mouse line. M.N., J.-P.C. and B.S.G. provided advice. F.K. wrote the original manuscript, which was reviewed and edited by D.A.D. and U.M. and edited by all other authors. U.M. supervised the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods, Supplementary Discussion, Supplementary Tables 1–3 and Supplementary Figures 1–11 (PDF 43421 kb)
Rights and permissions
About this article
Cite this article
Koukouli, F., Rooy, M., Tziotis, D. et al. Nicotine reverses hypofrontality in animal models of addiction and schizophrenia. Nat Med 23, 347–354 (2017). https://doi.org/10.1038/nm.4274
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4274
This article is cited by
-
CHRNA5 links chandelier cells to severity of amyloid pathology in aging and Alzheimer’s disease
Translational Psychiatry (2024)
-
Altered neuronal activity in the ventromedial prefrontal cortex drives nicotine intake escalation
Neuropsychopharmacology (2023)
-
Nicotine pretreatment alleviates MK-801-induced behavioral and cognitive deficits in mice by regulating Pdlim5/CRTC1 in the PFC
Acta Pharmacologica Sinica (2023)
-
Cocaine induces locomotor sensitization through a dopamine-dependent VTA-mPFC-FrA cortico-cortical pathway in male mice
Nature Communications (2023)
-
TA-GAN: transformer-driven addiction-perception generative adversarial network
Neural Computing and Applications (2023)