Abstract
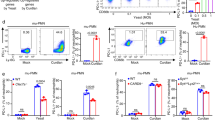
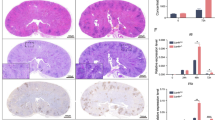
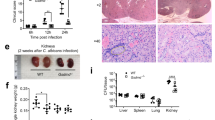
Opportunistic fungal infections are a leading cause of death among immune-compromised patients, and there is a pressing need to develop new antifungal therapeutic agents because of toxicity and resistance to the antifungal drugs currently in use. Although C-type lectin receptor– and Toll-like receptor–induced signaling pathways are key activators of host antifungal immunity, little is known about the mechanisms that negatively regulate host immune responses to a fungal infection. Here we found that JNK1 activation suppresses antifungal immunity in mice. We showed that JNK1-deficient mice had a significantly higher survival rate than wild-type control mice in response to Candida albicans infection, and the expression of JNK1 in hematopoietic innate immune cells was critical for this effect. JNK1 deficiency leads to significantly higher induction of CD23, a novel C-type lectin receptor, through NFATc1-mediated regulation of the CD23 gene promoter. Blocking either CD23 upregulation or CD23-dependent nitric oxide production eliminated the enhanced antifungal response found in JNK1-deficient mice. Notably, JNK inhibitors exerted potent antifungal therapeutic effects in both mouse and human cells infected with C. albicans, indicating that JNK1 may be a therapeutic target for treating fungal infection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Brown, G.D. et al. Hidden killers: human fungal infections. Sci. Transl. Med. 4, 165rv13 (2012).
Kim, J.Y. Human fungal pathogens: why should we learn? J. Microbiol. 54, 145–148 (2016).
Wüthrich, M., Deepe, G.S. Jr. & Klein, B. Adaptive immunity to fungi. Annu. Rev. Immunol. 30, 115–148 (2012).
Gow, N.A., van de Veerdonk, F.L., Brown, A.J. & Netea, M.G. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat. Rev. Microbiol. 10, 112–122 (2011).
Underhill, D.M. & Pearlman, E. Immune interactions with pathogenic and commensal fungi: a two-way street. Immunity 43, 845–858 (2015).
Plato, A., Hardison, S.E. & Brown, G.D. Pattern recognition receptors in antifungal immunity. Semin. Immunopathol. 37, 97–106 (2015).
Hardison, S.E. & Brown, G.D. C-type lectin receptors orchestrate antifungal immunity. Nat. Immunol. 13, 817–822 (2012).
Hoving, J.C., Wilson, G.J. & Brown, G.D. Signalling C-type lectin receptors, microbial recognition and immunity. Cell. Microbiol. 16, 185–194 (2014).
Brown, G.D. et al. Dectin-1 is a major β-glucan receptor on macrophages. J. Exp. Med. 196, 407–412 (2002).
Robinson, M.J. et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J. Exp. Med. 206, 2037–2051 (2009).
Zhu, L.L. et al. C-type lectin receptors dectin-3 and dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity 39, 324–334 (2013).
Saijo, S. et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat. Immunol. 8, 39–46 (2007).
Saijo, S. et al. Dectin-2 recognition of α-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32, 681–691 (2010).
Taylor, P.R. et al. Dectin-1 is required for β-glucan recognition and control of fungal infection. Nat. Immunol. 8, 31–38 (2007).
Dambuza, I.M. & Brown, G.D. C-type lectins in immunity: recent developments. Curr. Opin. Immunol. 32, 21–27 (2015).
Gross, O. et al. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 442, 651–656 (2006).
Zhu, L.L. et al. E3 ubiquitin ligase Cbl-b negatively regulates C-type lectin receptor–mediated antifungal innate immunity. J. Exp. Med. 213, 1555–1570 (2016).
Xiao, Y. et al. Targeting CBLB as a potential therapeutic approach for disseminated candidiasis. Nat. Med. 22, 906–914 (2016).
Wirnsberger, G. et al. Inhibition of CBLB protects from lethal Candida albicans sepsis. Nat. Med. 22, 915–923 (2016).
Dong, C., Davis, R.J. & Flavell, R.A. MAP kinases in the immune response. Annu. Rev. Immunol. 20, 55–72 (2002).
Wagner, E.F. & Nebreda, A.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 9, 537–549 (2009).
Arthur, J.S. & Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 13, 679–692 (2013).
Han, M.S. et al. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science 339, 218–222 (2013).
Bogoyevitch, M.A., Ngoei, K.R., Zhao, T.T., Yeap, Y.Y. & Ng, D.C. c-Jun N-terminal kinase (JNK) signaling: recent advances and challenges. Biochim. Biophys. Acta 1804, 463–475 (2010).
Davies, C. & Tournier, C. Exploring the function of the JNK (c-Jun N-terminal kinase) signalling pathway in physiological and pathological processes to design novel therapeutic strategies. Biochem. Soc. Trans. 40, 85–89 (2012).
Brown, G.D. Innate antifungal immunity: the key role of phagocytes. Annu. Rev. Immunol. 29, 1–21 (2011).
Fujiwara, H. et al. The absence of IgE antibody-mediated augmentation of immune responses in CD23-deficient mice. Proc. Natl. Acad. Sci. USA 91, 6835–6839 (1994).
Soilleux, E.J., Barten, R. & Trowsdale, J. DC-SIGN; a related gene, DC-SIGNR; and CD23 form a cluster on 19p13. J. Immunol. 165, 2937–2942 (2000).
Mossalayi, M.D. et al. CD23 mediates antimycobacterial activity of human macrophages. Infect. Immun. 77, 5537–5542 (2009).
Aubry, J.P. et al. The 25-kDa soluble CD23 activates type III constitutive nitric oxide-synthase activity via CD11b and CD11c expressed by human monocytes. J. Immunol. 159, 614–622 (1997).
Lecoanet-Henchoz, S. et al. CD23 regulates monocyte activation through a novel interaction with the adhesion molecules CD11b-CD18 and CD11c-CD18. Immunity 3, 119–125 (1995).
Vouldoukis, I. et al. IgE mediates killing of intracellular Toxoplasma gondii by human macrophages through CD23-dependent, interleukin-10 sensitive pathway. PLoS One 6, e18289 (2011).
Vouldoukis, I. et al. The killing of Leishmania major by human macrophages is mediated by nitric oxide induced after ligation of the FcɛRII/CD23 surface antigen. Proc. Natl. Acad. Sci. USA 92, 7804–7808 (1995).
Rambert, J. et al. Molecular blocking of CD23 supports its role in the pathogenesis of arthritis. PLoS One 4, e4834 (2009).
Wirnsberger, G. et al. Jagunal homolog 1 is a critical regulator of neutrophil function in fungal host defense. Nat. Genet. 46, 1028–1033 (2014).
Underhill, D.M., Rossnagle, E., Lowell, C.A. & Simmons, R.M. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 106, 2543–2550 (2005).
Vonk, A.G., Wieland, C.W., Netea, M.G. & Kullberg, B.J. Phagocytosis and intracellular killing of Candida albicans blastoconidia by neutrophils and macrophages: a comparison of different microbiological test systems. J. Microbiol. Methods 49, 55–62 (2002).
Romero-Puertas, M.C. & Sandalio, L.M. Nitric oxide level is self-regulating and also regulates its ROS partners. Front. Plant Sci. 7, 316 (2016).
Dong, C. et al. Defective T cell differentiation in the absence of Jnk1. Science 282, 2092–2095 (1998).
Debnath, I., Roundy, K.M., Weis, J.J. & Weis, J.H. Defining in vivo transcription factor complexes of the murine CD21 and CD23 genes. J. Immunol. 178, 7139–7150 (2007).
Kneitz, C. et al. The CD23b promoter is a target for NF-AT transcription factors in B-CLL cells. Biochim. Biophys. Acta 1588, 41–47 (2002).
Goodridge, H.S., Simmons, R.M. & Underhill, D.M. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J. Immunol. 178, 3107–3115 (2007).
Zanoni, I. & Granucci, F. Regulation and dysregulation of innate immunity by NFAT signaling downstream of pattern recognition receptors (PRRs). Eur. J. Immunol. 42, 1924–1931 (2012).
Bennett, B.L. et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 98, 13681–13686 (2001).
Lalaoui, N. et al. Targeting p38 or MK2 enhances the anti-leukemic activity of Smac-mimetics. Cancer Cell 29, 145–158 (2016).
Weston, C.R. & Davis, R.J. The JNK signal transduction pathway. Curr. Opin. Cell Biol. 19, 142–149 (2007).
Ersland, K., Wüthrich, M. & Klein, B.S. Dynamic interplay among monocyte-derived, dermal, and resident lymph node dendritic cells during the generation of vaccine immunity to fungi. Cell Host Microbe 7, 474–487 (2010).
Borregaard, N. Neutrophils, from marrow to microbes. Immunity 33, 657–670 (2010).
Zhao, X.Q. et al. C-type lectin receptor dectin-3 mediates trehalose 6,6′-dimycolate (TDM)-induced Mincle expression through CARD9/Bcl10/MALT1-dependent nuclear factor (NF)-κB activation. J. Biol. Chem. 289, 30052–30062 (2014).
Jia, X.M. et al. CARD9 mediates dectin-1-induced ERK activation by linking Ras-GRF1 to H-Ras for antifungal immunity. J. Exp. Med. 211, 2307–2321 (2014).
Sato, K. et al. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor γ chain to induce innate immune responses. J. Biol. Chem. 281, 38854–38866 (2006).
Flach, T.L. et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat. Med. 17, 479–487 (2011).
Acknowledgements
We thank X. Hu, Y. Shang and L. Ni for helpful discussion. We thank X. Xu, L. Mu, S. Xie and T. Xia for technical assistance. We thank D.C. Lin for editing the English in this manuscript. This work was partially supported by grants from the National Natural Science Foundation of China (81502460 and 31670904 to X.Z., 91542107 and 81630058 to X.L., 81571611 to X.J.) and National Institutes of Health (AI116722 to X.L.).
Author information
Authors and Affiliations
Contributions
X.Z. and Y.G. performed most of the experiments. C.J. and T.L. performed some of the mouse experiments. Q.C. and S.Z. performed some of the in vitro experiments. B.Z. analyzed the RNA–seq data and helped prepare the figures. X.J., M.-C.H. and C.D. provided key reagents and insightful discussion. X.L. and X.Z. conceived the project and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures and Table
Supplementary Figures 1–10 and Supplementary Tables 1 and 2 (PDF 5182 kb)
Rights and permissions
About this article
Cite this article
Zhao, X., Guo, Y., Jiang, C. et al. JNK1 negatively controls antifungal innate immunity by suppressing CD23 expression. Nat Med 23, 337–346 (2017). https://doi.org/10.1038/nm.4260
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4260
This article is cited by
-
Mitochondria-mediated ferroptosis induced by CARD9 ablation prevents MDSCs-dependent antifungal immunity
Cell Communication and Signaling (2024)
-
PU.1-CD23 signaling mediates pulmonary innate immunity against Aspergillus fumigatus infection by driving inflammatory response
BMC Immunology (2023)
-
Immune responses to human fungal pathogens and therapeutic prospects
Nature Reviews Immunology (2023)
-
Effects of TmTak1 silencing on AMP production as an Imd pathway component in Tenebrio molitor
Scientific Reports (2023)
-
CD146 deficiency promotes inflammatory type 2 responses in pulmonary cryptococcosis
Medical Microbiology and Immunology (2023)