Abstract
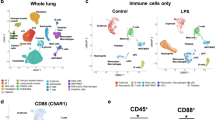
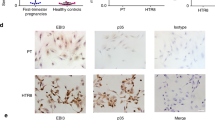
Preterm birth (PTB) is a leading cause of neonatal death worldwide1. Intrauterine and systemic infection and inflammation cause 30–40% of spontaneous preterm labor (PTL)2, which precedes PTB. Although antibody production is a major immune defense mechanism against infection, and B cell dysfunction has been implicated in pregnancy complications associated with PTL3,4, the functions of B cells in pregnancy are not well known5,6,7,8. We found that choriodecidua of women undergoing spontaneous PTL harbored functionally altered B cell populations. B cell–deficient mice were markedly more susceptible than wild-type (WT) mice to PTL after inflammation, but B cells conferred interleukin (IL)-10-independent protection against PTL. B cell deficiency in mice resulted in a lower uterine level of active progesterone-induced blocking factor 1 (PIBF1), and therapeutic administration of PIBF1 mitigated PTL and uterine inflammation in B cell–deficient mice. B cells are a significant producer of PIBF1 in human choriodecidua and mouse uterus in late gestation. PIBF1 expression by B cells is induced by the mucosal alarmin IL-33 (ref. 9). Human PTL was associated with diminished expression of the α-chain of IL-33 receptor on choriodecidual B cells and a lower level of active PIBF1 in late gestation choriodecidua. These results define a vital regulatory cascade involving IL-33, decidual B cells and PIBF1 in safeguarding term pregnancy and suggest new therapeutic approaches based on IL-33 and PIBF1 to prevent human PTL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Goldenberg, R.L., Culhane, J.F., Iams, J.D. & Romero, R. Epidemiology and causes of preterm birth. Lancet 371, 75–84 (2008).
Romero, R. et al. The preterm parturition syndrome. BJOG 113 (Suppl. 3), 17–42 (2006).
Jensen, F. et al. CD19+CD5+ cells as indicators of preeclampsia. Hypertension 59, 861–868 (2012).
Zhou, C.C. et al. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat. Med. 14, 855–862 (2008).
Matthiesen, L. et al. Immunology of preeclampsia. in Immunology of Pregnancy, Vol. 89 (ed. Markert, U.R.) 49–61 (Karger, Basel, 2005).
Frank, H.G. & Kaufmann, P. Nonvillous parts and trophoblast invasion. in Pathology of the Human Placenta (eds. Benirschke, K., Kaufmann, P. & Baergen, R.N.) 191–287 (Springer, New York, NY, 2006).
Trundley, A. & Moffett, A. Human uterine leukocytes and pregnancy. Tissue Antigens 63, 1–12 (2004).
Moffett, A. & Shreeve, N. First do no harm: uterine natural killer (NK) cells in assisted reproduction. Hum. Reprod. 30, 1519–1525 (2015).
Martin, N.T. & Martin, M.U. Interleukin 33 is a guardian of barriers and a local alarmin. Nat. Immunol. 17, 122–131 (2016).
Muzzio, D.O. et al. B cell development undergoes profound modifications and adaptations during pregnancy in mice. Biol. Reprod. 91, 115 (2014).
Zimmer, J.P., Garza, C., Butte, N.F. & Goldman, A.S. Maternal blood B-cell (CD19+) percentages and serum immunoglobulin concentrations correlate with breast-feeding behavior and serum prolactin concentration. Am. J. Reprod. Immunol. 40, 57–62 (1998).
Bhat, N.M., Mithal, A., Bieber, M.M., Herzenberg, L.A. & Teng, N.N. Human CD5+ B lymphocytes (B-1 cells) decrease in peripheral blood during pregnancy. J. Reprod. Immunol. 28, 53–60 (1995).
Medina, K.L. & Kincade, P.W. Pregnancy-related steroids are potential negative regulators of B lymphopoiesis. Proc. Natl. Acad. Sci. USA 91, 5382–5386 (1994).
Reiss, Y., Proudfoot, A.E., Power, C.A., Campbell, J.J. & Butcher, E.C. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J. Exp. Med. 194, 1541–1547 (2001).
Mora, J.R. et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature 424, 88–93 (2003).
Homey, B. et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat. Med. 8, 157–165 (2002).
Griffin, D.O., Holodick, N.E. & Rothstein, T.L. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70−. J. Exp. Med. 208, 67–80 (2011).
Litinskiy, M.B. et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3, 822–829 (2002).
Xu, W. et al. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat. Immunol. 8, 294–303 (2007).
Candando, K.M., Lykken, J.M. & Tedder, T.F. B10 cell regulation of health and disease. Immunol. Rev. 259, 259–272 (2014).
Rosser, E.C. & Mauri, C. Regulatory B cells: origin, phenotype, and function. Immunity 42, 607–612 (2015).
Robertson, S.A., Skinner, R.J. & Care, A.S. Essential role for IL-10 in resistance to lipopolysaccharide-induced preterm labor in mice. J. Immunol. 177, 4888–4896 (2006).
Thaxton, J.E., Romero, R. & Sharma, S. TLR9 activation coupled to IL-10 deficiency induces adverse pregnancy outcomes. J. Immunol. 183, 1144–1154 (2009).
Tian, J. et al. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J. Immunol. 167, 1081–1089 (2001).
Parekh, V.V. et al. B cells activated by lipopolysaccharide, but not by anti-Ig and anti-CD40 antibody, induce anergy in CD8+ T cells: role of TGF-beta 1. J. Immunol. 170, 5897–5911 (2003).
Shen, P. et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 507, 366–370 (2014).
Wang, R.X. et al. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat. Med. 20, 633–641 (2014).
Collison, L.W. et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450, 566–569 (2007).
Romero, R., Yeo, L., Chaemsaithong, P., Chaiworapongsa, T. & Hassan, S.S. Progesterone to prevent spontaneous preterm birth. Semin. Fetal Neonatal Med. 19, 15–26 (2014).
Szekeres-Bartho, J. & Polgar, B. PIBF: the double edged sword. Pregnancy and tumor. Am. J. Reprod. Immunol. 64, 77–86 (2010).
Hudić, I. et al. Maternal serum progesterone-induced blocking factor (PIBF) in the prediction of preterm birth. J. Reprod. Immunol. 109, 36–40 (2015).
Polgar, B. et al. Molecular cloning and immunologic characterization of a novel cDNA coding for progesterone-induced blocking factor. J. Immunol. 171, 5956–5963 (2003).
Lachmann, M. et al. PIBF (progesterone induced blocking factor) is overexpressed in highly proliferating cells and associated with the centrosome. Int. J. Cancer 112, 51–60 (2004).
Gonzalez-Arenas, A., Valadez-Cosmes, P., Jimenez-Arellano, C., Lopez-Sanchez, M. & Camacho-Arroyo, I. Progesterone-induced blocking factor is hormonally regulated in human astrocytoma cells, and increases their growth through the IL-4R/JAK1/STAT6 pathway. J. Steroid Biochem. Mol. Biol. 144 Pt B, 463–470 (2014).
Szekeres-Bartho, J. et al. The mechanism of the inhibitory effect of progesterone on lymphocyte cytotoxicity. I. Progesterone-treated lymphocytes release a substance inhibiting cytotoxicity and prostaglandin synthesis. Am. J. Reprod. Immunol. Microbiol. 9, 15–18 (1985).
Schiering, C. et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513, 564–568 (2014).
Sattler, S. et al. IL-10-producing regulatory B cells induced by IL-33 (Breg(IL-33)) effectively attenuate mucosal inflammatory responses in the gut. J. Autoimmun. 50, 107–122 (2014).
Burzyn, D. et al. A special population of regulatory T cells potentiates muscle repair. Cell 155, 1282–1295 (2013).
Bapat, S.P. et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature 528, 137–141 (2015).
Arpaia, N. et al. A distinct function of regulatory T cells in tissue protection. Cell 162, 1078–1089 (2015).
Chen, K. et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat. Immunol. 10, 889–898 (2009).
Acknowledgements
We thank the European Conditional Mouse Mutagenesis Program and Barry Rosen (Wellcome Trust Sanger Institute) for mouse Il33 targeting vectors, the NIAID Mucosal Immunology Studies Team (MIST) mouse core for generating the Il33−/− lacZ reporter mice, and M. Treadwell (University of Michigan at Ann Arbor) and X. Xu (Bristol-Myers Squibb) for discussion of the manuscript. This study was supported by grants from the Burroughs Wellcome Fund (Preterm Birth Initiative), US National Institutes of Health (U01AI95776 MIST Young Investigator Award, R21AI122256, P30CA22453), American Congress of Obstetricians and Gynecologists and Wayne State University (Perinatal Initiative) (to K.C.). A.N.F. was partially supported by a fellowship from the Wayne State University Office of the Vice President for Research.
Author information
Authors and Affiliations
Contributions
B.H. designed and performed the research, discussed and analyzed the data, and wrote the paper. A.N.F. designed and performed the research and analyzed the data. M.D.P., B.P., J.W.G., J.Z.Z., N.G.E.-H., J. Deng, J.L., F.Y., R.S.D., J.S.J., M.L.S., J. Dai, M.C., C.S. and L.A.P. performed the research and analyzed the data. R.A.N., T.B.J., M.H.B. and K.S.P. provided specimens. B.G., N.R.N., E.P. and W.-Z.W. revised the manuscript. A.C. and M.C. discussed the data. K.C. conceived the study, supervised and performed the research, discussed and analyzed the data, and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–3 and Supplementary Figures 1–15 (PDF 9252 kb)
Rights and permissions
About this article
Cite this article
Huang, B., Faucette, A., Pawlitz, M. et al. Interleukin-33-induced expression of PIBF1 by decidual B cells protects against preterm labor. Nat Med 23, 128–135 (2017). https://doi.org/10.1038/nm.4244
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4244