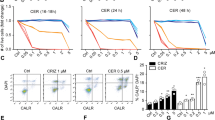
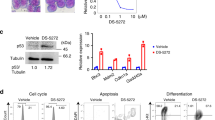
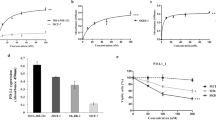
Abstract
The cellular inhibitors of apoptosis (cIAP) 1 and 2 are amplified in about 3% of cancers and have been identified in multiple malignancies as being potential therapeutic targets as a result of their role in the evasion of apoptosis. Consequently, small-molecule IAP antagonists, such as LCL161, have entered clinical trials for their ability to induce tumor necrosis factor (TNF)-mediated apoptosis of cancer cells. However, cIAP1 and cIAP2 are recurrently homozygously deleted in multiple myeloma (MM), resulting in constitutive activation of the noncanonical nuclear factor (NF)-κB pathway. To our surprise, we observed robust in vivo anti-myeloma activity of LCL161 in a transgenic myeloma mouse model and in patients with relapsed-refractory MM, where the addition of cyclophosphamide resulted in a median progression-free-survival of 10 months. This effect was not a result of direct induction of tumor cell death, but rather of upregulation of tumor-cell-autonomous type I interferon (IFN) signaling and a strong inflammatory response that resulted in the activation of macrophages and dendritic cells, leading to phagocytosis of tumor cells. Treatment of a MM mouse model with LCL161 established long-term anti-tumor protection and induced regression in a fraction of the mice. Notably, combination of LCL161 with the immune-checkpoint inhibitor anti-PD1 was curative in all of the treated mice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Gyrd-Hansen, M. & Meier, P. IAPs: from caspase inhibitors to modulators of NF-κB, inflammation and cancer. Nat. Rev. Cancer 10, 561–574 (2010).
Varfolomeev, E. et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-κB activation, and TNFα-dependent apoptosis. Cell 131, 669–681 (2007).
Vince, J.E. et al. IAP antagonists target cIAP1 to induce TNFα-dependent apoptosis. Cell 131, 682–693 (2007).
Petersen, S.L. et al. Autocrine TNFα signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell 12, 445–456 (2007).
Najem, S. et al. Smac mimetic LCL161 supports neuroblastoma chemotherapy in a drug class-dependent manner and synergistically interacts with ALK inhibitor TAE684 in cells with ALK mutation F1174L. Oncotarget http://dx.doi.org/10.18632/oncotarget.12055 (2016).
Chen, S.M. et al. Blockade of inhibitors of apoptosis proteins in combination with conventional chemotherapy leads to synergistic antitumor activity in medulloblastoma and cancer stem-like cells. PLoS One 11, e0161299 (2016).
Ramakrishnan, V. et al. Smac mimetic LCL161 overcomes protective ER stress induced by obatoclax, synergistically causing cell death in multiple myeloma. Oncotarget http://dx.doi.org/10.18632/oncotarget.11028 (2016).
Falkenhorst, J. et al. Inhibitor of apoptosis proteins (IAPs) are commonly dysregulated in GIST and can be pharmacologically targeted to enhance the pro-apoptotic activity of imatinib. Oncotarget http://dx.doi.org/10.18632/oncotarget.9159 (2016).
Shekhar, T.M. et al. IAP antagonists sensitize murine osteosarcoma cells to killing by TNFα. Oncotarget 7, 33866–33886 (2016).
Gerges, S., Rohde, K. & Fulda, S. Co-treatment with Smac mimetics and demethylating agents induces both apoptotic and necroptotic cell death pathways in acute lymphoblastic leukemia cells. Cancer Lett. 375, 127–132 (2016).
Tian, A. et al. Synergistic effects of IAP inhibitor LCL161 and paclitaxel on hepatocellular carcinoma cells. Cancer Lett. 351, 232–241 (2014).
Qin, Q. et al. Smac mimetic compound LCL161 sensitizes esophageal carcinoma cells to radiotherapy by inhibiting the expression of inhibitor of apoptosis protein. Tumour Biol. 35, 2565–2574 (2014).
Yuan, Z. et al. Blockade of inhibitors of apoptosis (IAPs) in combination with tumor-targeted delivery of tumor necrosis factor-α leads to synergistic antitumor activity. Cancer Gene Ther. 20, 46–56 (2013).
Chen, K.F. et al. Inhibition of Bcl-2 improves effect of LCL161, a SMAC mimetic, in hepatocellular carcinoma cells. Biochem. Pharmacol. 84, 268–277 (2012).
Weisberg, E. et al. Smac mimetics: implications for enhancement of targeted therapies in leukemia. Leukemia 24, 2100–2109 (2010).
Infante, J.R. et al. Phase I dose-escalation study of LCL161, an oral inhibitor of apoptosis proteins inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 32, 3103–3110 (2014).
Keats, J.J. et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell 12, 131–144 (2007).
Vallabhapurapu, S. et al. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat. Immunol. 9, 1364–1370 (2008).
Annunziata, C.M. et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell 12, 115–130 (2007).
Beug, S.T., Cheung, H.H., LaCasse, E.C. & Korneluk, R.G. Modulation of immune signalling by inhibitors of apoptosis. Trends Immunol. 33, 535–545 (2012).
Demchenko, Y.N. et al. Classical and/or alternative NF-κB pathway activation in multiple myeloma. Blood 115, 3541–3552 (2010).
Dougan, M. et al. IAP inhibitors enhance co-stimulation to promote tumor immunity. J. Exp. Med. 207, 2195–2206 (2010).
Knights, A.J., Fucikova, J., Pasam, A., Koernig, S. & Cebon, J. Inhibitor of apoptosis protein (IAP) antagonists demonstrate divergent immunomodulatory properties in human immune subsets with implications for combination therapy. Cancer Immunol. Immunother. 62, 321–335 (2013).
Müller-Sienerth, N. et al. SMAC mimetic BV6 induces cell death in monocytes and maturation of monocyte-derived dendritic cells. PLoS One 6, e21556 (2011).
Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, pl1 (2013).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Gardam, S. et al. Deletion of cIAP1 and cIAP2 in murine B lymphocytes constitutively activates cell survival pathways and inactivates the germinal center response. Blood 117, 4041–4051 (2011).
West, A.C. et al. The SMAC mimetic, LCL-161, reduces survival in aggressive MYC-driven lymphoma while promoting susceptibility to endotoxic shock. Oncogenesis 5, e216 (2016).
Chesi, M. et al. AID-dependent activation of a MYC transgene induces multiple myeloma in a conditional mouse model of post-germinal center malignancies. Cancer Cell 13, 167–180 (2008).
Petersen, S.L., Peyton, M., Minna, J.D. & Wang, X. Overcoming cancer cell resistance to Smac mimetic induced apoptosis by modulating cIAP-2 expression. Proc. Natl. Acad. Sci. USA 107, 11936–11941 (2010).
Beug, S.T. et al. Smac mimetics and innate immune stimuli synergize to promote tumor death. Nat. Biotechnol. 32, 182–190 (2014).
Yang, Y. et al. Targeting non-proteolytic protein ubiquitination for the treatment of diffuse large B cell lymphoma. Cancer Cell 29, 494–507 (2016).
Ramakrishnan, V. et al. Inhibitor of apoptosis proteins as therapeutic targets in multiple myeloma. Leukemia 28, 1519–1528 (2014).
Chauhan, D. et al. Targeting mitochondrial factor Smac/DIABLO as therapy for multiple myeloma (MM). Blood 109, 1220–1227 (2007).
Jourdan, M. et al. Tumor necrosis factor is a survival and proliferation factor for human myeloma cells. Eur. Cytokine Netw. 10, 65–70 (1999).
Chesi, M. et al. Drug response in a genetically engineered mouse model of multiple myeloma is predictive of clinical efficacy. Blood 120, 376–385 (2012).
Proietti, E. et al. Importance of cyclophosphamide-induced bystander effect on T cells for a successful tumor eradication in response to adoptive immunotherapy in mice. J. Clin. Invest. 101, 429–441 (1998).
Pallasch, C.P. et al. Sensitizing protective tumor microenvironments to antibody-mediated therapy. Cell 156, 590–602 (2014).
Christiansen, A.J. et al. Eradication of solid tumors using histone deacetylase inhibitors combined with immune-stimulating antibodies. Proc. Natl. Acad. Sci. USA 108, 4141–4146 (2011).
Kumar, S. et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 17, e328–e346 (2016).
Dewannieux, M., Dupressoir, A., Harper, F., Pierron, G. & Heidmann, T. Identification of autonomous IAP LTR retrotransposons mobile in mammalian cells. Nat. Genet. 36, 534–539 (2004).
Chiappinelli, K.B. et al. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell 162, 974–986 (2015).
Roulois, D. et al. DNA-demethylating agents target colorectal cancer cells by inducing viral mimicry by endogenous transcripts. Cell 162, 961–973 (2015).
Sistigu, A. et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat. Med. 20, 1301–1309 (2014).
Lecis, D. et al. Smac mimetics induce inflammation and necrotic tumour cell death by modulating macrophage activity. Cell Death Dis. 4, e920 (2013).
Haabeth, O.A. et al. Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer. Nat. Commun. 2, 240 (2011).
Boyman, O. & Sprent, J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol. 12, 180–190 (2012).
Obar, J.J. et al. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc. Natl. Acad. Sci. USA 107, 193–198 (2010).
Hope, C. et al. TPL2 kinase regulates the inflammatory milieu of the myeloma niche. Blood 123, 3305–3315 (2014).
Kim, J. et al. Macrophages and mesenchymal stromal cells support survival and proliferation of multiple myeloma cells. Br. J. Haematol. 158, 336–346 (2012).
Chao, M.P. et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 142, 699–713 (2010).
Pyonteck, S.M. et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 19, 1264–1272 (2013).
Beatty, G.L. et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331, 1612–1616 (2011).
Buhtoiarov, I.N. et al. Anti-tumour synergy of cytotoxic chemotherapy and anti-CD40 plus CpG-ODN immunotherapy through repolarization of tumour-associated macrophages. Immunology 132, 226–239 (2011).
Tveita, A.A. et al. Indirect CD4+ T-cell-mediated elimination of MHC II(NEG) tumor cells is spatially restricted and fails to prevent escape of antigen-negative cells. Eur. J. Immunol. 44, 2625–2637 (2014).
Beg, A.A. & Baltimore, D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science 274, 782–784 (1996).
Palumbo, A. et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N. Engl. J. Med. 366, 1759–1769 (2012).
Morgan, G.J. et al. Cyclophosphamide, thalidomide, and dexamethasone (CTD) as initial therapy for patients with multiple myeloma unsuitable for autologous transplantation. Blood 118, 1231–1238 (2011).
Baz, R.C. et al. Randomized multicenter phase 2 study of pomalidomide, cyclophosphamide, and dexamethasone in relapsed refractory myeloma. Blood 127, 2561–2568 (2016).
Moav, B. et al. Structure and activity of the translocated c-myc in mouse plasmacytoma XRPC-24. Gene 48, 297–300 (1986).
Müller, U. et al. Functional role of type I and type II interferons in antiviral defense. Science 264, 1918–1921 (1994).
Sato, M. et al. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-α/β gene induction. Immunity 13, 539–548 (2000).
Honda, K. et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434, 772–777 (2005).
Zaias, J., Mineau, M., Cray, C., Yoon, D. & Altman, N.H. Reference values for serum proteins of common laboratory rodent strains. J. Am. Assoc. Lab. Anim. Sci. 48, 387–390 (2009).
Pitt, L.A. et al. CXCL12-producing vascular endothelial niches control acute T cell leukemia maintenance. Cancer Cell 27, 755–768 (2015).
Waite, J.C. et al. Dynamic imaging of the effector immune response to listeria infection in vivo. PLoS Pathog. 7, e1001326 (2011).
Acknowledgements
We thank U. Jariwala, E. Slosberg and colleagues at Novartis for providing LCL161 and supporting the clinical trial. We are grateful to L. Rimsza for assistance with histological evaluation of splenocytes from LCL161 treated Vk*MYC mice and to F. Asimakopoulos for assistance with experimental design. We are indebted to M.S. Diamond (Washington University) for sharing IRF3/7null mice, to David Shealy from Centocor for donating the murine TNF blocking antibody and to P. Cohen (Mayo Clinic Arizona) for donating CD4, CD8 and NK1.1 blocking antibodies. M.C. was supported by research grants from the National Cancer Institute: CA190045 and CA186781.
Author information
Authors and Affiliations
Contributions
M.C. conceived the experiments. M.C., N.N.M., V.M.G., M.E.S., D.L.R. and N.K. executed the experiments. A.C.D. and H.E.K. provided statistical analysis of preclinical and clinical studies. Y.W.A. and P.L.B. performed bioinformatics analysis. A.C. contributed to the design of the study. G.J.A. processed clinical samples. K.M.M. coordinated patient accrual. R.F. supervised clinical sample processing and data acquisition. M.Q.L., D.D., S.K.K., S.A., A.D., F.B., M.A.G., C.B.R., Y.L., A.A.C.-K., A.K.S. and P.L.B. enrolled myeloma patients in the clinical trial. D.F. and I.A. designed and performed intra-vital microscopy studies. P.L.B. designed and conducted the clinical trial. M.C. and P.L.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.K.S. served as a consultant for Novartis. S.K.K. received research support from Novartis. The LCL161 clinical trial in MM patients was funded by Novartis.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11, Supplementary Tables 1–3, and Supplementary Data (PDF 7323 kb)
Rights and permissions
About this article
Cite this article
Chesi, M., Mirza, N., Garbitt, V. et al. IAP antagonists induce anti-tumor immunity in multiple myeloma. Nat Med 22, 1411–1420 (2016). https://doi.org/10.1038/nm.4229
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4229
This article is cited by
-
Immunogenic cell death in cancer: targeting necroptosis to induce antitumour immunity
Nature Reviews Cancer (2024)
-
LIGHT regulated gene expression in rheumatoid synovial fibroblasts
Molecular Biology Reports (2024)
-
Mitochondrial dysfunction and drug targets in multiple myeloma
Journal of Cancer Research and Clinical Oncology (2023)
-
Calorie restriction has no effect on bone marrow tumour burden in a Vk*MYC transplant model of multiple myeloma
Scientific Reports (2022)
-
Ultrafast prediction of somatic structural variations by filtering out reads matched to pan-genome k-mer sets
Nature Biomedical Engineering (2022)