Abstract
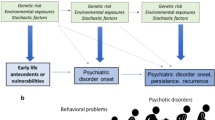
There is a growing understanding that pathological genetic variation and environmental insults during sensitive periods in brain development have long-term consequences on brain function, which range from learning disabilities to complex psychiatric disorders such as schizophrenia. Furthermore, recent experiments in animal models suggest that therapeutic interventions during sensitive periods, typically before the onset of clear neurological and behavioral symptoms, might prevent or ameliorate the development of specific pathologies. These studies suggest that understanding the dynamic nature of the pathophysiological mechanisms underlying psychiatric disorders is crucial for the development of effective therapies. In this Perspective, I explore the emerging concept of developmental windows in psychiatric disorders, their relevance for understanding disease progression and their potential for the design of new therapies. The limitations and caveats of early interventions in psychiatric disorders are also discussed in this context.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gore, F.M. et al. Global burden of disease in young people aged 10–24 years: a systematic analysis. Lancet 377, 2093–2102 (2011).
Lee, F.S. et al. Mental health. Adolescent mental health–opportunity and obligation. Science 346, 547–549 (2014).
van Os, J. & Kapur, S. Schizophrenia. Lancet 374, 635–645 (2009).
Jeste, S.S. & Geschwind, D.H. Clinical trials for neurodevelopmental disorders: At a therapeutic frontier. Sci. Transl. Med. 8, 321fs1 (2016).
Johnson, M.B. et al. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron 62, 494–509 (2009).
Jaffe, A.E. et al. Developmental regulation of human cortex transcription and its clinical relevance at single base resolution. Nat. Neurosci. 18, 154–161 (2015).
Hall, J., Trent, S., Thomas, K.L., O'Donovan, M.C. & Owen, M.J. Genetic risk for schizophrenia: convergence on synaptic pathways involved in plasticity. Biol. Psychiatry 77, 52–58 (2015).
Willsey, A.J. & State, M.W. Autism spectrum disorders: from genes to neurobiology. Curr. Opin. Neurobiol. 30, 92–99 (2015).
Sekar, A. et al. Schizophrenia risk from complex variation of complement component 4. Nature 530, 177–183 (2016).
Schmitt, A., Malchow, B., Hasan, A. & Falkai, P. The impact of environmental factors in severe psychiatric disorders. Front. Neurosci. 8, 19 (2014).
Hübener, M. & Bonhoeffer, T. Neuronal plasticity: beyond the critical period. Cell 159, 727–737 (2014).
Kalia, A. et al. Development of pattern vision following early and extended blindness. Proc. Natl. Acad. Sci. USA 111, 2035–2039 (2014).
Millan, M.J. et al. Altering the course of schizophrenia: progress and perspectives. Nat. Rev. Drug Discov. 15, 485–515 (2016).
Veenstra-VanderWeele, J. & Warren, Z. Intervention in the context of development: pathways toward new treatments. Neuropsychopharmacology 40, 225–237 (2015).
Silbereis, J.C., Pochareddy, S., Zhu, Y., Li, M. & Sestan, N. The cellular and molecular landscapes of the developing human central nervous system. Neuron 89, 248–268 (2016).
Wiesel, T.N. & Hubel, D.H. Extent of recovery from the effects of visual deprivation in kittens. J. Neurophysiol. 28, 1060–1072 (1965).
Greenhill, S.D. et al. Neurodevelopment. Adult cortical plasticity depends on an early postnatal critical period. Science 349, 424–427 (2015).
Anderson, P.J. & Reidy, N. Assessing executive function in preschoolers. Neuropsychol. Rev. 22, 345–360 (2012).
Friedmann, N. & Rusou, D. Critical period for first language: the crucial role of language input during the first year of life. Curr. Opin. Neurobiol. 35, 27–34 (2015).
Rakic, P., Bourgeois, J.P. & Goldman-Rakic, P.S. Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog. Brain Res. 102, 227–243 (1994).
Ebert, D.H. & Greenberg, M.E. Activity-dependent neuronal signalling and autism spectrum disorder. Nature 493, 327–337 (2013).
Penzes, P., Cahill, M.E., Jones, K.A., VanLeeuwen, J.E. & Woolfrey, K.M. Dendritic spine pathology in neuropsychiatric disorders. Nat. Neurosci. 14, 285–293 (2011).
Südhof, T.C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455, 903–911 (2008).
Marín, O. Interneuron dysfunction in psychiatric disorders. Nat. Rev. Neurosci. 13, 107–120 (2012).
Ben-Ari, Y. Excitatory actions of gaba during development: the nature of the nurture. Nat. Rev. Neurosci. 3, 728–739 (2002).
Ben-Ari, Y., Gaiarsa, J.L., Tyzio, R. & Khazipov, R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol. Rev. 87, 1215–1284 (2007).
Kirkby, L.A., Sack, G.S., Firl, A. & Feller, M.B. A role for correlated spontaneous activity in the assembly of neural circuits. Neuron 80, 1129–1144 (2013).
Uhlhaas, P.J., Roux, F., Rodriguez, E., Rotarska-Jagiela, A. & Singer, W. Neural synchrony and the development of cortical networks. Trends Cogn. Sci. 14, 72–80 (2010).
Tyzio, R. et al. Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science 314, 1788–1792 (2006).
Mwaniki, M.K., Atieno, M., Lawn, J.E. & Newton, C.R. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet 379, 445–452 (2012).
Ben-Ari, Y. Is birth a critical period in the pathogenesis of autism spectrum disorders? Nat. Rev. Neurosci. 16, 498–505 (2015).
Rochefort, N.L. et al. Sparsification of neuronal activity in the visual cortex at eye-opening. Proc. Natl. Acad. Sci. USA 106, 15049–15054 (2009).
Toyoizumi, T. et al. A theory of the transition to critical period plasticity: inhibition selectively suppresses spontaneous activity. Neuron 80, 51–63 (2013).
Nabel, E.M. & Morishita, H. Regulating critical period plasticity: insight from the visual system to fear circuitry for therapeutic interventions. Front. Psychiatry 4, 146 (2013).
Hubel, D.H. & Wiesel, T.N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J. Physiol. (Lond.) 206, 419–436 (1970).
Wang, B.S., Sarnaik, R. & Cang, J. Critical period plasticity matches binocular orientation preference in the visual cortex. Neuron 65, 246–256 (2010).
Hensch, T.K. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 6, 877–888 (2005).
Hu, H., Gan, J. & Jonas, P. Interneurons. Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science 345, 1255263 (2014).
Sigurdsson, T. & Duvarci, S. Hippocampal-prefrontal interactions in cognition, behavior and psychiatric disease. Front. Syst. Neurosci. 9, 190 (2016).
Leucht, S. et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 373, 31–41 (2009).
Green, M.F. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry 153, 321–330 (1996).
Weinberger, D.R. Implications of normal brain development for the pathogenesis of schizophrenia. Arch. Gen. Psychiatry 44, 660–669 (1987).
Rapoport, J.L., Giedd, J.N. & Gogtay, N. Neurodevelopmental model of schizophrenia: update 2012. Mol. Psychiatry 17, 1228–1238 (2012).
Insel, T.R. Rethinking schizophrenia. Nature 468, 187–193 (2010).
Wyatt, R.J. Neuroleptics and the natural course of schizophrenia. Schizophr. Bull. 17, 325–351 (1991).
Robinson, D. et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch. Gen. Psychiatry 56, 241–247 (1999).
Howes, O.D. et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch. Gen. Psychiatry 69, 776–786 (2012).
Honea, R., Crow, T.J., Passingham, D. & Mackay, C.E. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am. J. Psychiatry 162, 2233–2245 (2005).
Lewis, D.A., Curley, A.A., Glausier, J.R. & Volk, D.W. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 35, 57–67 (2012).
Lewis, D.A., Hashimoto, T. & Volk, D.W. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 6, 312–324 (2005).
Fusar-Poli, P. et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch. Gen. Psychiatry 69, 220–229 (2012).
Howes, O. et al. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Mol. Psychiatry 16, 885–886 (2011).
Fusar-Poli, P. et al. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci. Biobehav. Rev. 37, 1680–1691 (2013).
Davidson, M. et al. Behavioral and intellectual markers for schizophrenia in apparently healthy male adolescents. Am. J. Psychiatry 156, 1328–1335 (1999).
Lipska, B.K., Jaskiw, G.E. & Weinberger, D.R. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology 9, 67–75 (1993).
Modinos, G., Allen, P., Grace, A.A. & McGuire, P. Translating the MAM model of psychosis to humans. Trends Neurosci. 38, 129–138 (2015).
Jones, C.A., Watson, D.J. & Fone, K.C. Animal models of schizophrenia. Br. J. Pharmacol. 164, 1162–1194 (2011).
Yoon, K.J. et al. Modeling a genetic risk for schizophrenia in iPSCs and mice reveals neural stem cell deficits associated with adherens junctions and polarity. Cell Stem Cell 15, 79–91 (2014).
Shcheglovitov, A. et al. SHANK3 and IGF1 restore synaptic deficits in neurons from 22q13 deletion syndrome patients. Nature 503, 267–271 (2013).
Brennand, K.J. et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature 473, 221–225 (2011).
Kaiser, T. & Feng, G. Modeling psychiatric disorders for developing effective treatments. Nat. Med. 21, 979–988 (2015).
Richtand, N.M. et al. Risperidone pretreatment prevents elevated locomotor activity following neonatal hippocampal lesions. Neuropsychopharmacology 31, 77–89 (2006).
McGlashan, T.H. et al. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am. J. Psychiatry 163, 790–799 (2006).
Kim, I.H. et al. Spine pruning drives antipsychotic-sensitive locomotion via circuit control of striatal dopamine. Nat. Neurosci. 18, 883–891 (2015).
Kraguljac, N.V., White, D.M., Reid, M.A. & Lahti, A.C. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry 70, 1294–1302 (2013).
Schobel, S.A. et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 78, 81–93 (2013).
Brugger, S., Davis, J.M., Leucht, S. & Stone, J.M. Proton magnetic resonance spectroscopy and illness stage in schizophrenia--a systematic review and meta-analysis. Biol. Psychiatry 69, 495–503 (2011).
Del Pino, I. et al. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron 79, 1152–1168 (2013).
Andreou, C. et al. Increased resting-state gamma-band connectivity in first-episode schizophrenia. Schizophr. Bull. 41, 930–939 (2015).
Sun, L. et al. Evidence for dysregulated high-frequency oscillations during sensory processing in medication-naïve, first episode schizophrenia. Schizophr. Res. 150, 519–525 (2013).
Du, Y. & Grace, A.A. Peripubertal diazepam administration prevents the emergence of dopamine system hyperresponsivity in the MAM developmental disruption model of schizophrenia. Neuropsychopharmacology 38, 1881–1888 (2013).
Rudolph, U. & Knoflach, F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat. Rev. Drug Discov. 10, 685–697 (2011).
Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427 (2014).
Karayiorgou, M. et al. Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc. Natl. Acad. Sci. USA 92, 7612–7616 (1995).
Karayiorgou, M., Simon, T.J. & Gogos, J.A. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat. Rev. Neurosci. 11, 402–416 (2010).
Green, T. et al. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. J. Am. Acad. Child Adolesc. Psychiatry 48, 1060–1068 (2009).
Sigurdsson, T., Stark, K.L., Karayiorgou, M., Gogos, J.A. & Gordon, J.A. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature 464, 763–767 (2010).
Mukai, J. et al. Molecular substrates of altered axonal growth and brain connectivity in a mouse model of schizophrenia. Neuron 86, 680–695 (2015).
Tamura, M., Mukai, J., Gordon, J.A. & Gogos, J.A. Developmental inhibition of Gsk3 rescues behavioral and neurophysiological deficits in a mouse model of schizophrenia predisposition. Neuron 89, 1100–1109 (2016).
Do, K.Q. et al. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur. J. Neurosci. 12, 3721–3728 (2000).
Steullet, P. et al. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J. Neurosci. 30, 2547–2558 (2010).
Cabungcal, J.H. et al. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron 83, 1073–1084 (2014).
Behrens, M.M. et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science 318, 1645–1647 (2007).
Morishita, H., Cabungcal, J.H., Chen, Y., Do, K.Q. & Hensch, T.K. Prolonged period of cortical plasticity upon redox dysregulation in fast-spiking interneurons. Biol. Psychiatry 78, 396–402 (2015).
Farokhnia, M. et al. N-acetylcysteine as an adjunct to risperidone for treatment of negative symptoms in patients with chronic schizophrenia: a randomized, double-blind, placebo-controlled study. Clin. Neuropharmacol. 36, 185–192 (2013).
Do, K.Q., Cabungcal, J.H., Frank, A., Steullet, P. & Cuenod, M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr. Opin. Neurobiol. 19, 220–230 (2009).
Koseki, T. et al. Exposure to enriched environments during adolescence prevents abnormal behaviours associated with histone deacetylation in phencyclidine-treated mice. Int. J. Neuropsychopharmacol. 15, 1489–1501 (2012).
Lee, H. et al. Early cognitive experience prevents adult deficits in a neurodevelopmental schizophrenia model. Neuron 75, 714–724 (2012).
Hagerman, R.J. et al. Advances in the treatment of fragile X syndrome. Pediatrics 123, 378–390 (2009).
Penagarikano, O., Mulle, J.G. & Warren, S.T. The pathophysiology of fragile X syndrome. Annu. Rev. Genomics Hum. Genet. 8, 109–129 (2007).
Santoro, M.R., Bray, S.M. & Warren, S.T. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annu. Rev. Pathol. 7, 219–245 (2012).
Bear, M.F., Huber, K.M. & Warren, S.T. The mGluR theory of fragile X mental retardation. Trends Neurosci. 27, 370–377 (2004).
Garber, K.B., Visootsak, J. & Warren, S.T. Fragile X syndrome. Eur. J. Hum. Genet. 16, 666–672 (2008).
Bureau, I., Shepherd, G.M. & Svoboda, K. Circuit and plasticity defects in the developing somatosensory cortex of FMR1 knock-out mice. J. Neurosci. 28, 5178–5188 (2008).
Cruz-Martín, A., Crespo, M. & Portera-Cailliau, C. Delayed stabilization of dendritic spines in fragile X mice. J. Neurosci. 30, 7793–7803 (2010).
Galvez, R. & Greenough, W.T. Sequence of abnormal dendritic spine development in primary somatosensory cortex of a mouse model of the fragile X mental retardation syndrome. Am. J. Med. Genet. A. 135, 155–160 (2005).
Harlow, E.G. et al. Critical period plasticity is disrupted in the barrel cortex of FMR1 knockout mice. Neuron 65, 385–398 (2010).
Nimchinsky, E.A., Oberlander, A.M. & Svoboda, K. Abnormal development of dendritic spines in FMR1 knock-out mice. J. Neurosci. 21, 5139–5146 (2001).
Dölen, G. et al. Correction of fragile X syndrome in mice. Neuron 56, 955–962 (2007).
Kim, H., Gibboni, R., Kirkhart, C. & Bao, S. Impaired critical period plasticity in primary auditory cortex of fragile X model mice. J. Neurosci. 33, 15686–15692 (2013).
Till, S.M. et al. Altered maturation of the primary somatosensory cortex in a mouse model of fragile X syndrome. Hum. Mol. Genet. 21, 2143–2156 (2012).
Berry-Kravis, E. et al. Seizures in fragile X syndrome: characteristics and comorbid diagnoses. Am. J. Intellect. Dev. Disabil. 115, 461–472 (2010).
Musumeci, S.A. et al. Audiogenic seizures susceptibility in transgenic mice with fragile X syndrome. Epilepsia 41, 19–23 (2000).
Gonçalves, J.T., Anstey, J.E., Golshani, P. & Portera-Cailliau, C. Circuit level defects in the developing neocortex of Fragile X mice. Nat. Neurosci. 16, 903–909 (2013).
Gibson, J.R., Bartley, A.F., Hays, S.A. & Huber, K.M. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J. Neurophysiol. 100, 2615–2626 (2008).
Sourdet, V., Russier, M., Daoudal, G., Ankri, N. & Debanne, D. Long-term enhancement of neuronal excitability and temporal fidelity mediated by metabotropic glutamate receptor subtype 5. J. Neurosci. 23, 10238–10248 (2003).
Braat, S. & Kooy, R.F. The GABAA receptor as a therapeutic target for neurodevelopmental disorders. Neuron 86, 1119–1130 (2015).
He, Q., Nomura, T., Xu, J. & Contractor, A. The developmental switch in GABA polarity is delayed in fragile X mice. J. Neurosci. 34, 446–450 (2014).
Tyzio, R. et al. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science 343, 675–679 (2014).
de Vrij, F.M. et al. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol. Dis. 31, 127–132 (2008).
Michalon, A. et al. Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron 74, 49–56 (2012).
Su, T. et al. Early continuous inhibition of group 1 mGlu signaling partially rescues dendritic spine abnormalities in the Fmr1 knockout mouse model for fragile X syndrome. Psychopharmacology (Berl.) 215, 291–300 (2011).
Yan, Q.J., Rammal, M., Tranfaglia, M. & Bauchwitz, R.P. Suppression of two major Fragile X syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology 49, 1053–1066 (2005).
Berry-Kravis, E. et al. Mavoglurant in fragile X syndrome: Results of two randomized, double-blind, placebo-controlled trials. Sci. Transl. Med. 8, 321ra5 (2016).
Scharf, S.H., Jaeschke, G., Wettstein, J.G. & Lindemann, L. Metabotropic glutamate receptor 5 as drug target for Fragile X syndrome. Curr. Opin. Pharmacol. 20, 124–134 (2015).
Berry-Kravis, E.M. et al. Effects of STX209 (arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome: a randomized, controlled, phase 2 trial. Sci. Transl. Med. 4, 152ra127 (2012).
Lemonnier, E. et al. A randomised controlled trial of bumetanide in the treatment of autism in children. Transl. Psychiatry 2, e202 (2012).
Hadjikhani, N. et al. Improving emotional face perception in autism with diuretic bumetanide: a proof-of-concept behavioral and functional brain imaging pilot study. Autism 19, 149–157 (2015).
Marguet, S.L. et al. Treatment during a vulnerable developmental period rescues a genetic epilepsy. Nat. Med. 21, 1436–1444 (2015).
Chahrour, M. & Zoghbi, H.Y. The story of Rett syndrome: from clinic to neurobiology. Neuron 56, 422–437 (2007).
Hagberg, B. Clinical manifestations and stages of Rett syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 8, 61–65 (2002).
Ausió, J., Martínez de Paz, A. & Esteller, M. MeCP2: the long trip from a chromatin protein to neurological disorders. Trends Mol. Med. 20, 487–498 (2014).
Katz, D.M. et al. Rett syndrome: crossing the threshold to clinical translation. Trends Neurosci. 39, 100–113 (2016).
Lombardi, L.M., Baker, S.A. & Zoghbi, H.Y. MECP2 disorders: from the clinic to mice and back. J. Clin. Invest. 125, 2914–2923 (2015).
Durand, S. et al. NMDA receptor regulation prevents regression of visual cortical function in the absence of Mecp2. Neuron 76, 1078–1090 (2012).
Krishnan, K. et al. MeCP2 regulates the timing of critical period plasticity that shapes functional connectivity in primary visual cortex. Proc. Natl. Acad. Sci. USA 112, E4782–E4791 (2015).
Mierau, S.B., Patrizi, A., Hensch, T.K. & Fagiolini, M. Cell-specific regulation of N-methyl-D-aspartate receptor maturation by Mecp2 in cortical circuits. Biol. Psychiatry 79, 746–754 (2016).
Huang, Z.J. et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98, 739–755 (1999).
Dani, V.S. et al. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. USA 102, 12560–12565 (2005).
Moghaddam, B. & Krystal, J.H. Capturing the angel in “angel dust”: twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophr. Bull. 38, 942–949 (2012).
Patrizi, A. et al. Chronic administration of the N-methyl-D-aspartate receptor antagonist ketamine improves Rett syndrome phenotype. Biol. Psychiatry 79, 755–764 (2016).
Kron, M. et al. Brain activity mapping in Mecp2 mutant mice reveals functional deficits in forebrain circuits, including key nodes in the default mode network, that are reversed with ketamine treatment. J. Neurosci. 32, 13860–13872 (2012).
Ehninger, D., Li, W., Fox, K., Stryker, M.P. & Silva, A.J. Reversing neurodevelopmental disorders in adults. Neuron 60, 950–960 (2008).
McGraw, C.M., Samaco, R.C. & Zoghbi, H.Y. Adult neural function requires MeCP2. Science 333, 186 (2011).
Green, J. et al. Intervention for infants at risk of developing autism: a case series. J. Autism Dev. Disord. 43, 2502–2514 (2013).
Miklowitz, D.J. et al. Family-focused treatment for adolescents and young adults at high risk for psychosis: results of a randomized trial. J. Am. Acad. Child Adolesc. Psychiatry 53, 848–858 (2014).
Larroque, B. et al. Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. Lancet 371, 813–820 (2008).
Lin, A. et al. Outcomes of nontransitioned cases in a sample at ultra-high risk for psychosis. Am. J. Psychiatry 172, 249–258 (2015).
Bousman, C.A. et al. Effects of NRG1 and DAOA genetic variation on transition to psychosis in individuals at ultra-high risk for psychosis. Transl. Psychiatry 3, e251 (2013).
Corcoran, C.M. et al. HPA axis function and symptoms in adolescents at clinical high risk for schizophrenia. Schizophr. Res. 135, 170–174 (2012).
Perkins, D.O. et al. Towards a psychosis risk blood diagnostic for persons experiencing high-risk symptoms: preliminary results from the NAPLS project. Schizophr. Bull. 41, 419–428 (2015).
Egerton, A., Fusar-Poli, P. & Stone, J.M. Glutamate and psychosis risk. Curr. Pharm. Des. 18, 466–478 (2012).
Tognin, S. et al. Using structural neuroimaging to make quantitative predictions of symptom progression in individuals at ultra-high risk for psychosis. Front. Psychiatry 4, 187 (2014).
Bodatsch, M., Brockhaus-Dumke, A., Klosterkötter, J. & Ruhrmann, S. Forecasting psychosis by event-related potentials-systematic review and specific meta-analysis. Biol. Psychiatry 77, 951–958 (2015).
Wolff, J.J. et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am. J. Psychiatry 169, 589–600 (2012).
Jones, W. & Klin, A. Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature 504, 427–431 (2013).
Maayan, L. & Correll, C.U. Weight gain and metabolic risks associated with antipsychotic medications in children and adolescents. J. Child Adolesc. Psychopharmacol. 21, 517–535 (2011).
Ikonomidou, C. et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 283, 70–74 (1999).
Bittigau, P. et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc. Natl. Acad. Sci. USA 99, 15089–15094 (2002).
Brambrink, A.M. et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology 112, 834–841 (2010).
Schneider, M. et al. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Am. J. Psychiatry 171, 627–639 (2014).
Kessler, R.C. et al. Age of onset of mental disorders: a review of recent literature. Curr. Opin. Psychiatry 20, 359–364 (2007).
Kessler, R.C. et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization's World Mental Health Survey Initiative. World Psychiatry 6, 168–176 (2007).
Pratt, J., Winchester, C., Dawson, N. & Morris, B. Advancing schizophrenia drug discovery: optimizing rodent models to bridge the translational gap. Nat. Rev. Drug Discov. 11, 560–579 (2012).
Dobbs, D. Schizophrenia: the making of a troubled mind. Nature 468, 154–156 (2010).
Le Magueresse, C. & Monyer, H. GABAergic interneurons shape the functional maturation of the cortex. Neuron 77, 388–405 (2013).
Acknowledgements
I apologize for not citing all relevant references, owing to space limitations. I thank B. Rico and members of the Marín lab for valuable discussions and their critical reading of this manuscript. The European Research Council (ERC-2011-AdG 293683), Wellcome Trust (103714MA), Simons Foundation Autism Research Initiative (SFARI 239766OM) and De Spoelberch Foundation support work on this topic in my laboratory. O.M. is a Wellcome Trust Investigator.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Marín, O. Developmental timing and critical windows for the treatment of psychiatric disorders. Nat Med 22, 1229–1238 (2016). https://doi.org/10.1038/nm.4225
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4225
This article is cited by
-
N-acetylcysteine during critical neurodevelopmental periods prevents behavioral and neurochemical deficits in the Poly I:C rat model of schizophrenia
Translational Psychiatry (2024)
-
Converging synaptic and network dysfunctions in distinct autoimmune encephalitis
EMBO Reports (2024)
-
An epigenetic barrier sets the timing of human neuronal maturation
Nature (2024)
-
The Challenge of Accounting for the Moderator Effect of Risk Exposure on the Effectiveness of Mindfulness-Based Treatments for Youth
International Journal of Applied Positive Psychology (2024)
-
Assessing a multivariate model of brain-mediated genetic influences on disordered eating in the ABCD cohort
Nature Mental Health (2023)