Abstract
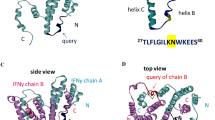
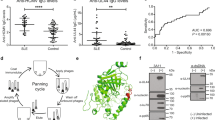
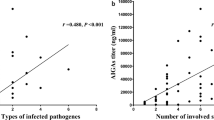
The binding of autoantibodies (autoAbs) to interferon (IFN)-γ in people with mycobacterial diseases has become an emerging medical concern. Many patients display specific human leukocyte antigen (HLA) class II haplotypes, which suggests that a common T cell–dependent and B cell–dependent mechanism might underlie the production of specific anti-IFN-γ autoAbs. We show here that these autoAbs target a major epitope (amino acids 121–131, designated position (P)121–131) in a region crucial for IFN-γ receptor (IFN-γR) activation to impair IFN-γ-mediated activities. The amino acid sequence of this epitope is highly homologous to a stretch in the Noc2 protein of Aspergillus spp., which was cross-reactive with autoAbs from patients. Rats immunized with Aspergillus Noc2 developed antibodies that reacted with human IFN-γ. We generated an epitope-erased variant of IFN-γ (EE-IFN-γ), in which the major neutralizing epitope region was altered. The binding affinity of anti-IFN-γ autoAbs for EE-IFN-γ was reduced by about 40%, as compared to that for IFN-γ1–131. Moreover, EE-IFN-γ activated the IFN-γR downstream signaling pathway ex vivo, irrespectively of anti-IFN-γ autoAbs. In conclusion, we identified a common, crucial B cell epitope that bound to anti-IFN-γ autoAbs in patients, and we propose a molecular-mimicry model for autoAb development. In addition, treatment with EE-IFN-γ might be worth investigating in patients producing anti-IFN-γ autoAbs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Döffinger, R. et al. Autoantibodies to interferon-γ in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin. Infect. Dis. 38, e10–e14 (2004).
Höflich, C. et al. Naturally occurring anti-IFN-γ autoantibody and severe infections with Mycobacterium cheloneae and Burkholderia cocovenenans. Blood 103, 673–675 (2004).
Kampmann, B. et al. Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-γ. J. Clin. Invest. 115, 2480–2488 (2005).
Patel, S.Y. et al. Anti-IFN-γ autoantibodies in disseminated nontuberculous mycobacterial infections. J. Immunol. 175, 4769–4776 (2005).
Puel, A. et al. Recurrent staphylococcal cellulitis and subcutaneous abscesses in a child with autoantibodies against IL-6. J. Immunol. 180, 647–654 (2008).
Puel, A. et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J. Exp. Med. 207, 291–297 (2010).
Browne, S.K. et al. Adult-onset immunodeficiency in Thailand and Taiwan. N. Engl. J. Med. 367, 725–734 (2012).
Chi, C.Y. et al. Anti-IFN-γ autoantibodies in adults with disseminated nontuberculous mycobacterial infections are associated with HLA-DRB1*16:02 and HLA-DQB1*05:02 and the reactivation of latent varicella-zoster virus infection. Blood 121, 1357–1366 (2013).
Rosen, L.B. et al. Anti-GM-CSF autoantibodies in patients with cryptococcal meningitis. J. Immunol. 190, 3959–3966 (2013).
Lee, W.I. et al. Patients with inhibitory and neutralizing auto-antibodies to interferon-γ resemble the sporadic adult-onset phenotype of mendelian susceptibility to mycobacterial disease (MSMD) lacking bacille Calmette-Guerin (BCG)-induced diseases. Immunobiology 218, 762–771 (2013).
Browne, S.K. Anticytokine autoantibody-associated immunodeficiency. Annu. Rev. Immunol. 32, 635–657 (2014).
Rosen, L.B. et al. Nocardia-induced granulocyte macrophage colony-stimulating factor is neutralized by autoantibodies in disseminated/extrapulmonary nocardiosis. Clin. Infect. Dis. 60, 1017–1025 (2015).
Chi, C.Y. et al. Clinical manifestations, course, and outcome of patients with neutralizing anti-interferon-γ autoantibodies and disseminated nontuberculous mycobacterial infections. Medicine (Baltimore) 95, e3927 (2016).
Levin, M. et al. Familial disseminated atypical mycobacterial infection in childhood: a human mycobacterial susceptibility gene? Lancet 345, 79–83 (1995).
Casanova, J.L. et al. Idiopathic disseminated bacillus Calmette-Guérin infection: a French national retrospective study. Pediatrics 98, 774–778 (1996).
Bustamante, J., Boisson-Dupuis, S., Abel, L. & Casanova, J.L. Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-γ immunity. Semin. Immunol. 26, 454–470 (2014).
Puel, A. & Casanova, J.L. Autoantibodies against cytokines: back to human genetics. Blood 121, 1246–1247 (2013).
Sfriso, P. et al. Infections and autoimmunity: the multifaceted relationship. J. Leukoc. Biol. 87, 385–395 (2010).
Rowley, D. & Jenkin, C.R. Antigenic cross-reaction between host and parasite as a possible cause of pathogenicity. Nature 193, 151–154 (1962).
Cusick, M.F., Libbey, J.E. & Fujinami, R.S. Molecular mimicry as a mechanism of autoimmune disease. Clin. Rev. Allergy Immunol. 42, 102–111 (2012).
van den Berg, B. et al. Guillain-Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat. Rev. Neurol. 10, 469–482 (2014).
Kain, R. et al. Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat. Med. 14, 1088–1096 (2008).
Ku, C.L. et al. Anti-IFN-γ autoantibodies are strongly associated with HLA-DR*15:02/16:02 and HLA-DQ*05:01/05:02 across Southeast Asia. J. Allergy Clin. Immunol. 137, 945–948.e8 (2016).
Emini, E.A., Hughes, J.V., Perlow, D.S. & Boger, J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J. Virol. 55, 836–839 (1985).
Larsen, J.E., Lund, O. & Nielsen, M. Improved method for predicting linear B-cell epitopes. Immunome Res. 2, 2 (2006).
Bordoli, L. et al. Protein structure homology modeling using SWISS-MODEL workspace. Nat. Protoc. 4, 1–13 (2009).
Ealick, S.E. et al. Three-dimensional structure of recombinant human interferon-γ. Science 252, 698–702 (1991).
Walter, M.R. et al. Crystal structure of a complex between interferon-γ and its soluble high-affinity receptor. Nature 376, 230–235 (1995).
Thiel, D.J. et al. Observation of an unexpected third receptor molecule in the crystal structure of human interferon-γ receptor complex. Structure 8, 927–936 (2000).
Lundell, D. et al. The carboxyl-terminal region of human interferon gamma is important for biological activity: mutagenic and NMR analysis. Protein Eng. 4, 335–341 (1991).
Savan, R., Ravichandran, S., Collins, J.R., Sakai, M. & Young, H.A. Structural conservation of interferon γ among vertebrates. Cytokine Growth Factor Rev. 20, 115–124 (2009).
Basham, T.Y. & Merigan, T.C. Recombinant interferon-γ increases HLA-DR synthesis and expression. J. Immunol. 130, 1492–1494 (1983).
Seelig, G.F., Wijdenes, J., Nagabhushan, T.L. & Trotta, P.P. Evidence for a polypeptide segment at the carboxyl terminus of recombinant human gamma interferon involved in expression of biological activity. Biochemistry 27, 1981–1987 (1988).
Wang, Y. et al. Characterization of pathogenic human monoclonal autoantibodies against GM-CSF. Proc. Natl. Acad. Sci. USA 110, 7832–7837 (2013).
Piccoli, L. et al. Neutralization and clearance of GM-CSF by autoantibodies in pulmonary alveolar proteinosis. Nat. Commun. 6, 7375 (2015).
Baxter, C.G. et al. Performance of two Aspergillus IgG EIA assays compared with the precipitin test in chronic and allergic aspergillosis. Clin. Microbiol. Infect. 19, E197–E204 (2013).
Barnes, P.D. & Marr, K.A. Aspergillosis: spectrum of disease, diagnosis, and treatment. Infect. Dis. Clin. North Am. 20, 545–561 (2006).
Nguyen, L.D., Viscogliosi, E. & Delhaes, L. The lung mycobiome: an emerging field of the human respiratory microbiome. Front. Microbiol. 6, 89 (2015).
Chaudhary, N., Staab, J.F. & Marr, K.A. Healthy human T-Cell Responses to Aspergillus fumigatus antigens. PLoS One 5, e9036 (2010).
Oldstone, M.B. Molecular mimicry and immune-mediated diseases. FASEB J. 12, 1255–1265 (1998).
Neal, C.O. et al. Global population structure of Aspergillus terreus inferred by ISSR typing reveals geographical subclustering. BMC Microbiol. 11, 203 (2011).
Browne, S.K. et al. Anti-CD20 (rituximab) therapy for anti-IFN-γ autoantibody-associated nontuberculous mycobacterial infection. Blood 119, 3933–3939 (2012).
Rao, V.K. et al. Use of rituximab for refractory cytopenias associated with autoimmune lymphoproliferative syndrome (ALPS). Pediatr. Blood Cancer 52, 847–852 (2009).
Keating, G.M. Rituximab: a review of its use in chronic lymphocytic leukaemia, low-grade or follicular lymphoma and diffuse large B-cell lymphoma. Drugs 70, 1445–1476 (2010).
Sologuren, I. et al. Partial recessive IFN-γR1 deficiency: genetic, immunological and clinical features of 14 patients from 11 kindreds. Hum. Mol. Genet. 20, 1509–1523 (2011).
Smith, K. et al. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat. Protoc. 4, 372–384 (2009).
Acknowledgements
We thank all the clinicians and patients who participated in this study. This work was supported by the Taiwan National Health Research Institutes grant NHRI-EX100-10028SC (C.-L.K.), Taiwan Ministry of Science and Technology grant 100-2314-B-182-050 (C.-L.K.), 102-2320-B-182-024 (C.-L.K.) and 103-2320-B-182-012 (C.-L.K.), and Chang Gung Memorial Hospital grant CMRPD1F0201 (C.-L.K.), CORPD1F0041 (C.-L.K.) and BMRPB98 (C.-L.K.).
Author information
Authors and Affiliations
Contributions
C.-H. Lin and C.-L.K. designed the study and analyzed the data, and wrote the manuscript; C.-H. Lin., C.-Y.C., H.-P.S., J.-Y.D. and C.-C.L. performed the research and analyzed the data; C.-F.Y., M.-W.H. and C.-H. Lee referred the patients and designed the study; S.-Y.W., C.-Y.K., K.-H.T., S.-H.L., H.-K.C., C.-H.H. and H.-C.L. analyzed the data; H.-K.C. and C.-H.H. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
A patent application concerning EE-IFN-γ has been submitted by C.-H. Lin, C.-Y.C., J.-Y.D., H.-P.S. and C.-L.K. on the basis of these results.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 and Supplementary Table 1 (PDF 6602 kb)
Rights and permissions
About this article
Cite this article
Lin, CH., Chi, CY., Shih, HP. et al. Identification of a major epitope by anti-interferon-γ autoantibodies in patients with mycobacterial disease. Nat Med 22, 994–1001 (2016). https://doi.org/10.1038/nm.4158
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4158
This article is cited by
-
The detectable anti-interferon-γ autoantibodies in COVID-19 patients may be associated with disease severity
Virology Journal (2023)
-
Characteristics and Outcomes of Anti-interferon Gamma Antibody-Associated Adult Onset Immunodeficiency
Journal of Clinical Immunology (2023)
-
Talaromyces marneffei and Mycobacterium tuberculosis co-infection in a patient with high titer anti-interferon-γ autoantibodies: a case report
BMC Infectious Diseases (2022)
-
Clinical findings and predictive factors for positive anti-interferon-γ autoantibodies in patients suffering from a non-tuberculosis mycobacteria or Talaromyces marneffei infection: a multicenter prospective cohort study
Scientific Reports (2022)
-
Determination of a distinguished interferon gamma epitope recognized by monoclonal antibody relating to autoantibody associated immunodeficiency
Scientific Reports (2022)