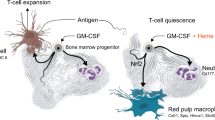
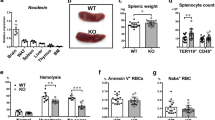
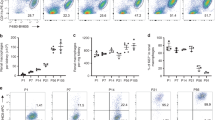
Abstract
Iron is an essential component of the erythrocyte protein hemoglobin and is crucial to oxygen transport in vertebrates. In the steady state, erythrocyte production is in equilibrium with erythrocyte removal1. In various pathophysiological conditions, however, erythrocyte life span is compromised severely, which threatens the organism with anemia and iron toxicity2,3. Here we identify an on-demand mechanism that clears erythrocytes and recycles iron. We show that monocytes that express high levels of lymphocyte antigen 6 complex, locus C1 (LY6C1, also known as Ly-6C) ingest stressed and senescent erythrocytes, accumulate in the liver via coordinated chemotactic cues, and differentiate into ferroportin 1 (FPN1, encoded by SLC40A1)-expressing macrophages that can deliver iron to hepatocytes. Monocyte-derived FPN1+Tim-4neg macrophages are transient, reside alongside embryonically derived T cell immunoglobulin and mucin domain containing 4 (Timd4, also known as Tim-4)high Kupffer cells (KCs), and depend on the growth factor Csf1 and the transcription factor Nrf2 (encoded by Nfe2l2). The spleen, likewise, recruits iron-loaded Ly-6Chigh monocytes, but these do not differentiate into iron-recycling macrophages, owing to the suppressive action of Csf2. The accumulation of a transient macrophage population in the liver also occurs in mouse models of hemolytic anemia, anemia of inflammation, and sickle cell disease. Inhibition of monocyte recruitment to the liver during stressed erythrocyte delivery leads to kidney and liver damage. These observations identify the liver as the primary organ that supports rapid erythrocyte removal and iron recycling, and uncover a mechanism by which the body adapts to fluctuations in erythrocyte integrity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Hentze, M.W., Muckenthaler, M.U., Galy, B. & Camaschella, C. Two to tango: regulation of mammalian iron metabolism. Cell 142, 24–38 (2010).
Theurl, I. et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood 113, 5277–5286 (2009).
Weinberg, E.D. The hazards of iron loading. Metallomics 2, 732–740 (2010).
Ganz, T. Macrophages and systemic iron homeostasis. J. Innate Immun. 4, 446–453 (2012).
Higgins, J.M. & Mahadevan, L. Physiological and pathological population dynamics of circulating human red blood cells. Proc. Natl. Acad. Sci. USA 107, 20587–20592 (2010).
Weiss, G. & Goodnough, L.T. Anemia of chronic disease. N. Engl. J. Med. 352, 1011–1023 (2005).
Luten, M. et al. Survival of red blood cells after transfusion: a comparison between red cells concentrates of different storage periods. Transfusion 48, 1478–1485 (2008).
Prasad, A.S. et al. Hereditary sideroblastic anemia and glucose-6-phosphate dehydrogenase deficiency in a Negro family. J. Clin. Invest. 47, 1415–1424 (1968).
Tinmouth, A. et al. Clinical consequences of red cell storage in the critically ill. Transfusion 46, 2014–2027 (2006).
Wilairat, P., Kittikalayawong, A. & Chaicharoen, S. The thalassemic red cell membrane. Southeast Asian J. Trop. Med. Public Health 23 (Suppl. 2), 74–78 (1992).
Knutson, M.D., Oukka, M., Koss, L.M., Aydemir, F. & Wessling-Resnick, M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc. Natl. Acad. Sci. USA 102, 1324–1328 (2005).
Soares, M.P. & Hamza, I. Macrophages and iron metabolism. Immunity 44, 492–504 (2016).
Hod, E.A. et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood 115, 4284–4292 (2010).
Zocchi, E. et al. Hepatic or splenic targeting of carrier erythrocytes: a murine model. Biotechnol. Appl. Biochem. 9, 423–434 (1987).
Terpstra, V. & van Berkel, T.J. Scavenger receptors on liver Kupffer cells mediate the in vivo uptake of oxidatively damaged red blood cells in mice. Blood 95, 2157–2163 (2000).
Nemeth, E. et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306, 2090–2093 (2004).
Haldar, M. et al. Heme-mediated SPI-C induction promotes monocyte differentiation into iron-recycling macrophages. Cell 156, 1223–1234 (2014).
Kohyama, M. et al. Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature 457, 318–321 (2009).
Gardenghi, S. et al. Distinct roles for hepcidin and interleukin-6 in the recovery from anemia in mice injected with heat-killed Brucella abortus. Blood 123, 1137–1145 (2014).
Sasu, B.J. et al. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood 115, 3616–3624 (2010).
Ryan, T.M., Ciavatta, D.J. & Townes, T.M. Knockout-transgenic mouse model of sickle cell disease. Science 278, 873–876 (1997).
Gautier, E.L. et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 13, 1118–1128 (2012).
Robbins, C.S. et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 19, 1166–1172 (2013).
Goldmann, T. et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci. 16, 1618–1626 (2013).
Yona, S. et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 (2013).
Geissmann, F. et al. Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 (2010).
Sarrazin, S. & Sieweke, M. Integration of cytokine and transcription factor signals in hematopoietic stem cell commitment. Semin. Immunol. 23, 326–334 (2011).
Marro, S. et al. Heme controls ferroportin1 (FPN1) transcription involving Bach1, Nrf2 and a MARE/ARE sequence motif at position -7007 of the FPN1 promoter. Haematologica 95, 1261–1268 (2010).
Nairz, M. et al. Nitric oxide-mediated regulation of ferroportin-1 controls macrophage iron homeostasis and immune function in Salmonella infection. J. Exp. Med. 210, 855–873 (2013).
Kovtunovych, G., Eckhaus, M.A., Ghosh, M.C., Ollivierre-Wilson, H. & Rouault, T.A. Dysfunction of the heme recycling system in heme oxygenase 1-deficient mice: effects on macrophage viability and tissue iron distribution. Blood 116, 6054–6062 (2010).
Zhang, Z. et al. Ferroportin1 deficiency in mouse macrophages impairs iron homeostasis and inflammatory responses. Blood 118, 1912–1922 (2011).
Swirski, F.K. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 (2009).
Theurl, M. et al. Kupffer cells modulate iron homeostasis in mice via regulation of hepcidin expression. J Mol Med (Berl) 86, 825–835 (2008).
Ross, S.L. et al. Molecular mechanism of hepcidin-mediated ferroportin internalization requires ferroportin lysines, not tyrosines or JAK-STAT. Cell Metab. 15, 905–917 (2012).
Ludwiczek, S. et al. Ca2+ channel blockers reverse iron overload by a new mechanism via divalent metal transporter-1. Nat. Med. 13, 448–454 (2007).
Torrance, J.D. & Bothwell, T.H. A simple technique for measuring storage iron concentrations in formalinised liver samples. S. Afr. J. Med. Sci. 33, 9–11 (1968).
Grundy, M.A., Gorman, N., Sinclair, P.R., Chorney, M.J. & Gerhard, G.S. High-throughput non-heme iron assay for animal tissues. J. Biochem. Biophys. Methods 59, 195–200 (2004).
Rainer, J., Sanchez-Cabo, F., Stocker, G., Sturn, A. & Trajanoski, Z. CARMAweb: comprehensive R- and bioconductor-based web service for microarray data analysis. Nucleic Acids Res. 34, W498–W503 (2006).
Sturn, A., Quackenbush, J. & Trajanoski, Z. Genesis: cluster analysis of microarray data. Bioinformatics 18, 207–208 (2002).
Acknowledgements
This work was supported in part by US National Institutes of Health (NIH) grants 1R01HL095612, R01HL128264, R56AI104695 and the Massachusetts General Hospital's Howard M. Goodman Fellowship (to F.K.S.) and R01DK071837 (to H.Y.L.). I.T. was supported by the Max Kade Foundation and grants by the Austrian Science Fund (FWF) (P28302-B30 and P24749-B13)). I.H. (HI 1573/1-1, HI 1573/2-1) and G.F.W. were supported by the German Research Foundation. M. Nairz was supported by an FWF Erwin Schroedinger Fellowship (J3486-B13). L.M.S.G. and N.K.H. were supported by the Boehringer Ingelheim Fonds. F.W. was supported by the National Natural Science Foundation of China (31530034 and 31225013). We thank D. Capen for help in electron microscopy; N. Bonheur and M. Waring for help with cell sorting; A. Lindau for technical assistance; C. Haerdtner, J. Kornemann, and J. Zou for help with flow cytometry; D. Brown for interpretation of electron microscopy images; C. Robbins for the mouse drawing; and K. Joyes for editing the manuscript.
Author information
Authors and Affiliations
Contributions
I.T., I.H., and M. Nairz conceived the project, designed and performed experiments, analyzed and interpreted data, and wrote the manuscript; P.T., D.H., M.A., S.H., L.M.S.G., T.A.W.H., M.S., S.S., A.M.F., A.A., S.R., C.M., M.T., P.W., Y.I., G.F.W., N.K.H., B.G.C. and M.M. performed experiments; T.L.A., F.W., M.P. and P.W. provided materials and intellectual input; O.M.D.L., J.L.B., M. Nahrendorf, and G.W. provided intellectual input and edited the manuscript; L.B. and E.R. conceived and conducted the clinical trial. R.W. edited the manuscript; H.Y.L. conceived the project, helped interpret data, and edited the manuscript; F.K.S. conceived the project, designed experiments, analyzed and interpreted data, and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–18 and Supplementary Table 1 (PDF 12632 kb)
Rights and permissions
About this article
Cite this article
Theurl, I., Hilgendorf, I., Nairz, M. et al. On-demand erythrocyte disposal and iron recycling requires transient macrophages in the liver. Nat Med 22, 945–951 (2016). https://doi.org/10.1038/nm.4146
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4146
This article is cited by
-
The roles of tissue resident macrophages in health and cancer
Experimental Hematology & Oncology (2024)
-
A secretory protein neudesin regulates splenic red pulp macrophages in erythrophagocytosis and iron recycling
Communications Biology (2024)
-
Physiology and diseases of tissue-resident macrophages
Nature (2023)
-
Targeting macrophages in hematological malignancies: recent advances and future directions
Journal of Hematology & Oncology (2022)
-
Nanomaterials: small particles show huge possibilities for cancer immunotherapy
Journal of Nanobiotechnology (2022)