Abstract
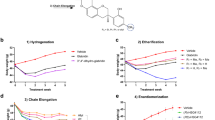
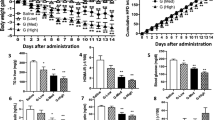
The increasing global prevalence of obesity and its associated disorders points to an urgent need for the development of novel and effective therapeutic strategies that induce healthy weight loss. Obesity is characterized by hyperleptinemia and central leptin resistance. In an attempt to identify compounds that could reverse leptin resistance and thus promote weight loss, we analyzed a library of small molecules that have mRNA expression profiles similar to that of celastrol, a naturally occurring compound that we previously identified as a leptin sensitizer. Through this process, we identified another naturally occurring compound, withaferin A, that also acts as a leptin sensitizer. We found that withaferin-A treatment of mice with diet-induced obesity (DIO) resulted in a 20–25% reduction of body weight, while also decreasing obesity-associated abnormalities, including hepatic steatosis. Withaferin-A treatment marginally affected the body weight of ob/ob and db/db mice, both of which are deficient in leptin signaling. In addition, withaferin A, unlike celastrol, has beneficial effects on glucose metabolism that occur independently of its leptin-sensitizing effect. Our results show that the metabolic abnormalities of DIO can be mitigated by sensitizing animals to endogenous leptin, and they indicate that withaferin A is a potential leptin sensitizer with additional antidiabetic actions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Wang, Y., Beydoun, M.A., Liang, L., Caballero, B. & Kumanyika, S.K. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 16, 2323–2330 (2008).
Olshansky, S.J. et al. A potential decline in life expectancy in the United States in the 21st century. N. Engl. J. Med. 352, 1138–1145 (2005).
Berrington de Gonzalez, A. et al. Body-mass index and mortality among 1.46 million white adults. N. Engl. J. Med. 363, 2211–2219 (2010).
Rodgers, R.J., Tschöp, M.H. & Wilding, J.P. Anti-obesity drugs: past, present and future. Dis. Model. Mech. 5, 621–626 (2012).
Dalamaga, M. et al. Leptin at the intersection of neuroendocrinology and metabolism: current evidence and therapeutic perspectives. Cell Metab. 18, 29–42 (2013).
Myers, M.G. Jr. et al. Challenges and opportunities of defining clinical leptin resistance. Cell Metab. 15, 150–156 (2012).
Dietrich, M.O. & Horvath, T.L. Hypothalamic control of energy balance: insights into the role of synaptic plasticity. Trends Neurosci. 36, 65–73 (2013).
Friedman, J.M. Modern science versus the stigma of obesity. Nat. Med. 10, 563–569 (2004).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homolog. Nature 372, 425–432 (1994).
Frederich, R.C. et al. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat. Med. 1, 1311–1314 (1995).
Considine, R.V. et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 334, 292–295 (1996).
Maffei, M. et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1, 1155–1161 (1995).
Heymsfield, S.B. et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. J. Am. Med. Assoc. 282, 1568–1575 (1999).
Mori, H. et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat. Med. 10, 739–743 (2004).
Howard, J.K. et al. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat. Med. 10, 734–738 (2004).
Loh, K. et al. Elevated hypothalamic TCPTP in obesity contributes to cellular leptin resistance. Cell Metab. 14, 684–699 (2011).
Horvath, T.L. et al. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc. Natl. Acad. Sci. USA 107, 14875–14880 (2010).
Schneeberger, M. et al. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell 155, 172–187 (2013).
Dietrich, M.O., Liu, Z.W. & Horvath, T.L. Mitochondrial dynamics controlled by mitofusins regulate Agrp neuronal activity and diet-induced obesity. Cell 155, 188–199 (2013).
Diano, S. et al. Peroxisome proliferation-associated control of reactive oxygen species sets melanocortin tone and feeding in diet-induced obesity. Nat. Med. 17, 1121–1127 (2011).
Long, L., Toda, C., Jeong, J.K., Horvath, T.L. & Diano, S. PPAR-γ ablation sensitizes proopiomelanocortin neurons to leptin during high-fat feeding. J. Clin. Invest. 124, 4017–4027 (2014).
Ren, D., Li, M., Duan, C. & Rui, L. Identification of SH2-B as a key regulator of leptin sensitivity, energy balance and body weight in mice. Cell Metab. 2, 95–104 (2005).
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 (2007).
Park, S.W. & Ozcan, U. Potential for therapeutic manipulation of the UPR in disease. Semin. Immunopathol. 35, 351–373 (2013).
Lee, J. & Ozcan, U. Unfolded protein response signaling and metabolic diseases. J. Biol. Chem. 289, 1203–1211 (2014).
Ozcan, U. et al. Endoplasmic reticulum stress links obesity, insulin action and type 2 diabetes. Science 306, 457–461 (2004).
Ozcan, U. et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140 (2006).
Ozcan, L. et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 9, 35–51 (2009).
Ramírez, S. & Claret, M. Hypothalamic ER stress: a bridge between leptin resistance and obesity. FEBS Lett. 589, 1678–1687 (2015).
Won, J.C. et al. Central administration of an endoplasmic reticulum stress inducer inhibits the anorexigenic effects of leptin and insulin. Obesity (Silver Spring) 17, 1861–1865 (2009).
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T. & Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 (2001).
Calfon, M. et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP1 mRNA. Nature 415, 92–96 (2002).
Williams, K.W. et al. Xbp1s in Pomc neurons connects ER stress with energy balance and glucose homeostasis. Cell Metab. 20, 471–482 (2014).
Lamb, J. et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes and disease. Science 313, 1929–1935 (2006).
Liu, J., Lee, J., Salazar Hernandez, M.A., Mazitschek, R. & Ozcan, U. Treatment of obesity with celastrol. Cell 161, 999–1011 (2015).
Mirjalili, M.H., Moyano, E., Bonfill, M., Cusido, R.M. & Palazón, J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules 14, 2373–2393 (2009).
Winters, M. Ancient medicine, modern use: Withania somnifera and its potential role in integrative oncology. Altern. Med. Rev. 11, 269–277 (2006).
Mishra, L.C., Singh, B.B. & Dagenais, S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern. Med. Rev. 5, 334–346 (2000).
Halaas, J.L. et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–546 (1995).
Van Heek, M. et al. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J. Clin. Invest. 99, 385–390 (1997).
Halaas, J.L. et al. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc. Natl. Acad. Sci. USA 94, 8878–8883 (1997).
Pelleymounter, M.A. et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269, 540–543 (1995).
Campfield, L.A., Smith, F.J., Guisez, Y., Devos, R. & Burn, P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269, 546–549 (1995).
Schwartz, M.W., Seeley, R.J., Campfield, L.A., Burn, P. & Baskin, D.G. Identification of targets of leptin action in rat hypothalamus. J. Clin. Invest. 98, 1101–1106 (1996).
Fei, H. et al. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc. Natl. Acad. Sci. USA 94, 7001–7005 (1997).
Hâkansson, M.L., Brown, H., Ghilardi, N., Skoda, R.C. & Meister, B. Leptin receptor immunoreactivity in chemically defined target neurons of the hypothalamus. J. Neurosci. 18, 559–572 (1998).
Ozcan, U. et al. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol. Cell 29, 541–551 (2008).
Yu, Y. et al. Withaferin A targets heat-shock protein 90 in pancreatic cancer cells. Biochem. Pharmacol. 79, 542–551 (2010).
Neckers, L. Heat-shock protein 90: the cancer chaperone. J. Biosci. 32, 517–530 (2007).
Kaileh, M. et al. Withaferin a strongly elicits IκB kinase beta hyperphosphorylation concomitant with potent inhibition of its kinase activity. J. Biol. Chem. 282, 4253–4264 (2007).
Harris, R.B., Kelso, E.W., Flatt, W.P., Bartness, T.J. & Grill, H.J. Energy expenditure and body composition of chronically maintained decerebrate rats in the fed and fasted condition. Endocrinology 147, 1365–1376 (2006).
Arch, J.R., Stock, M.J. & Trayhurn, P. Leptin resistance in obese humans: does it exist and what does it mean? Int. J. Obes. Relat. Metab. Disord. 22, 1159–1163 (1998).
Ma, X. et al. Celastrol protects against obesity and metabolic dysfunction through activation of a HSF1–PGC-1α transcriptional axis. Cell Metab. 22, 695–708 (2015).
Denver, R.J., Bonett, R.M. & Boorse, G.C. Evolution of leptin structure and function. Neuroendocrinology 94, 21–38 (2011).
Kwiatkowski, D.J. et al. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1-null cells. Hum. Mol. Genet. 11, 525–534 (2002).
Zavacki, A.M. et al. Type 1 iodothyronine deiodinase is a sensitive marker of peripheral thyroid status in the mouse. Endocrinology 146, 1568–1575 (2005).
Kaplan, M.M. Subcellular alterations causing reduced hepatic thyroxine-5′-monodeiodinase activity in fasted fats. Endocrinology 104, 58–64 (1979).
Acknowledgements
We greatly appreciate M. White for his time spent on critical reading of our manuscript and for his contributions and suggestions. We thank S. Cabi and I. Cakir for their initial experimental contributions to this work. We also thank S. Mert and B. Akosman for their help on some of the experiments, and S. Huang and M. Mulcahey-Maynard for measurement of serum T3 levels. TSC+/+ and TSC−/− MEFs were kindly provided by D. Kwiatkowski (Dana-Farber Cancer Institute). This work was mainly supported by funds provided to U.O. from the Department of Medicine, Boston Children's Hospital, and also by grant R01DK098496 (U.O.) from the US National Institutes of Health and American Diabetes Association Career Development grant #7-09-CD-10 (U.O.).
Author information
Authors and Affiliations
Contributions
U.O. came up with the idea to investigate compounds that have similar gene expression to celastrol by using CMAP. U.O. directed this and subsequent analyses and picked withaferin A as a candidate for the treatment of type 2 diabetes and obesity. J. Lee, J. Liu, X.F., M.A.S.H., P.M., D.I. and J.W.C. performed experiments under the direction of U.O. U.O., J. Lee, J. Liu and X.F. analyzed data. U.O. wrote the manuscript, and J. Lee, J. Liu and X.F. were involved in the writing and preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
U.O. is a scientific founder, shareholder, and scientific advisory board and board of directors member of ERX Pharmaceuticals.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 1870 kb)
Rights and permissions
About this article
Cite this article
Lee, J., Liu, J., Feng, X. et al. Withaferin A is a leptin sensitizer with strong antidiabetic properties in mice. Nat Med 22, 1023–1032 (2016). https://doi.org/10.1038/nm.4145
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4145
This article is cited by
-
Identification of an autophagy-related gene signature for predicting prognosis and immune activity in pancreatic adenocarcinoma
Scientific Reports (2022)
-
Identification of a c-MYB-directed therapeutic for acute myeloid leukemia
Leukemia (2022)
-
Anti-obesity drug discovery: advances and challenges
Nature Reviews Drug Discovery (2022)
-
Intragastric safflower yellow and its main component HSYA improve leptin sensitivity before body weight change in diet-induced obese mice
Naunyn-Schmiedeberg's Archives of Pharmacology (2022)
-
Prediction of drug efficacy from transcriptional profiles with deep learning
Nature Biotechnology (2021)