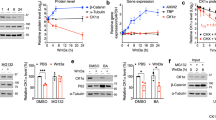
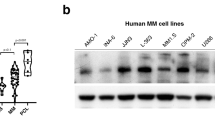
Abstract
Immunomodulatory drugs (IMiDs), such as thalidomide and its derivatives lenalidomide and pomalidomide, are key treatment modalities for hematologic malignancies, particularly multiple myeloma (MM) and del(5q) myelodysplastic syndrome (MDS). Cereblon (CRBN), a substrate receptor of the CRL4 ubiquitin ligase complex, is the primary target by which IMiDs mediate anticancer and teratogenic effects. Here we identify a ubiquitin-independent physiological chaperone-like function of CRBN that promotes maturation of the basigin (BSG; also known as CD147) and solute carrier family 16 member 1 (SLC16A1; also known as MCT1) proteins. This process allows for the formation and activation of the CD147–MCT1 transmembrane complex, which promotes various biological functions, including angiogenesis, proliferation, invasion and lactate export. We found that IMiDs outcompete CRBN for binding to CD147 and MCT1, leading to destabilization of the CD147–MCT1 complex. Accordingly, IMiD-sensitive MM cells lose CD147 and MCT1 expression after being exposed to IMiDs, whereas IMiD-resistant cells retain their expression. Furthermore, del(5q) MDS cells have elevated CD147 expression, which is attenuated after IMiD treatment. Finally, we show that BSG (CD147) knockdown phenocopies the teratogenic effects of thalidomide exposure in zebrafish. These findings provide a common mechanistic framework to explain both the teratogenic and pleiotropic antitumor effects of IMiDs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Mellin, G.W. & Katzenstein, M. The saga of thalidomide. Neuropathy to embryopathy, with case reports of congenital anomalies. N. Engl. J. Med. 267, 1238–1244 (1962).
Shortt, J., Hsu, A.K. & Johnstone, R.W. Thalidomide-analog biology: immunological, molecular and epigenetic targets in cancer therapy. Oncogene 32, 4191–4202 (2013).
Bartlett, J.B., Dredge, K. & Dalgleish, A.G. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat. Rev. Cancer 4, 314–322 (2004).
Ito, T. et al. Identification of a primary target of thalidomide teratogenicity. Science 327, 1345–1350 (2010).
Zhu, Y.X. et al. Cereblon expression is required for the anti-myeloma activity of lenalidomide and pomalidomide. Blood 118, 4771–4779 (2011).
Lopez-Girona, A. et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 26, 2326–2335 (2012).
Lee, J. & Zhou, P. DCAFs, the missing link of the CUL4–DDB1 ubiquitin ligase. Mol. Cell 26, 775–780 (2007).
Angers, S. et al. Molecular architecture and assembly of the DDB1–CUL4A ubiquitin ligase machinery. Nature 443, 590–593 (2006).
Fischer, E.S. et al. Structure of the DDB1–CRBN E3 ubiquitin ligase in complex with thalidomide. Nature 512, 49–53 (2014).
Chamberlain, P.P. et al. Structure of the human cereblon–DDB1–lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nat. Struct. Mol. Biol. 21, 803–809 (2014).
Jo, S., Lee, K.H., Song, S., Jung, Y.K. & Park, C.S. Identification and functional characterization of cereblon as a binding protein for large-conductance calcium-activated potassium channels in rat brain. J. Neurochem. 94, 1212–1224 (2005).
Lee, K.M., Jo, S., Kim, H., Lee, J. & Park, C.S. Functional modulation of AMP-activated protein kinase by cereblon. Biochim. Biophys. Acta 1813, 448–455 (2011).
Iacono, K.T., Brown, A.L., Greene, M.I. & Saouaf, S.J. CD147 immunoglobulin superfamily receptor function and role in pathology. Exp. Mol. Pathol. 83, 283–295 (2007).
Kirk, P. et al. CD147 is tightly associated with lactate transporters MCT1 and MCT4, and facilitates their cell surface expression. EMBO J. 19, 3896–3904 (2000).
Parks, S.K., Chiche, J. & Pouysségur, J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat. Rev. Cancer 13, 611–623 (2013).
Walters, D.K., Arendt, B.K. & Jelinek, D.F. CD147 regulates the expression of MCT1 and lactate export in multiple myeloma cells. Cell Cycle 12, 3175–3183 (2013).
Arendt, B.K. et al. Increased expression of extracellular matrix metalloproteinase inducer (CD147) in multiple myeloma: role in regulation of myeloma cell proliferation. Leukemia 26, 2286–2296 (2012).
Zhu, D. et al. The cyclophilin A–CD147 complex promotes the proliferation and homing of multiple-myeloma cells. Nat. Med. 21, 572–580 (2015).
Krönke, J. et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple-myeloma cells. Science 343, 301–305 (2014).
Lu, G. et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 343, 305–309 (2014).
Gandhi, A.K. et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4CRBN. Br. J. Haematol. 164, 811–821 (2014).
Zhu, Y.X. et al. Identification of cereblon-binding proteins and relationship with response and survival after IMiDs in multiple myeloma. Blood 124, 536–545 (2014).
Krönke, J. et al. Lenalidomide induces ubiquitination and degradation of CK1-α in del(5q) MDS. Nature 523, 183–188 (2015).
Petzold, G., Fischer, E.S. & Thomä, N.H. Structural basis of lenalidomide-induced CK1-α degradation by the CRL4CRBN ubiquitin ligase. Nature 532, 127–130 (2016).
Chesi, M. et al. Drug response in a genetically engineered mouse model of multiple myeloma is predictive of clinical efficacy. Blood 120, 376–385 (2012).
Smith, C.K., Baker, T.A. & Sauer, R.T. Lon and Clp family proteases and chaperones share homologous substrate-recognition domains. Proc. Natl. Acad. Sci. USA 96, 6678–6682 (1999).
Gandhi, A.K. et al. Measuring cereblon as a biomarker of response or resistance to lenalidomide and pomalidomide requires use of standardized reagents and understanding of gene complexity. Br. J. Haematol. 164, 233–244 (2014).
Tang, Y. et al. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res. 65, 3193–3199 (2005).
Strupp, C., Hildebrandt, B., Germing, U., Haas, R. & Gattermann, N. Cytogenetic response to thalidomide treatment in three patients with myelodysplastic syndrome. Leukemia 17, 1200–1202 (2003).
Kelaidi, C. et al. Treatment of myelodysplastic syndromes with 5q deletion before the lenalidomide era; the GFM experience with EPO and thalidomide. Leuk. Res. 32, 1049–1053 (2008).
Fenaux, P. et al. A randomized phase 3 study of lenalidomide versus placebo in RBC-transfusion-dependent patients with low- or intermediate-1-risk myelodysplastic syndromes with del5q. Blood 118, 3765–3776 (2011).
List, A. et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N. Engl. J. Med. 355, 1456–1465 (2006).
Giagounidis, A. et al. Lenalidomide as a disease-modifying agent in patients with del(5q) myelodysplastic syndromes: linking mechanism of action to clinical outcomes. Ann. Hematol. 93, 1–11 (2014).
Mahony, C. et al. Pomalidomide is nonteratogenic in chicken and zebrafish embryos and non-neurotoxic in vitro. Proc. Natl. Acad. Sci. USA 110, 12703–12708 (2013).
Bassermann, F., Eichner, R. & Pagano, M. The ubiquitin–proteasome system—implications for cell cycle control and the targeted treatment of cancer. Biochim. Biophys. Acta 1843, 150–162 (2014).
Kumar, S.K. et al. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol. 15, 1503–1512 (2014).
Roussel, M. et al. Front-line transplantation program with lenalidomide, bortezomib and dexamethasone combination as induction and consolidation, followed by lenalidomide maintenance in patients with multiple myeloma: a phase 2 study by the Intergroupe Francophone du Myélome. J. Clin. Oncol. 32, 2712–2717 (2014).
Richardson, P.G. et al. Lenalidomide, bortezomib and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood 116, 679–686 (2010).
Stewart, A.K. et al. Carfilzomib, lenalidomide and dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 372, 142–152 (2015).
Harrison, J.S., Rameshwar, P., Chang, V. & Bandari, P. Oxygen saturation in the bone marrow of healthy volunteers. Blood 99, 394 (2002).
Staffler, G. et al. Selective inhibition of T cell activation via CD147 through novel modulation of lipid rafts. J. Immunol. 171, 1707–1714 (2003).
Hu, J. et al. Involvement of HAb18G–CD147 in T cell activation and immunological synapse formation. J. Cell. Mol. Med. 14, 2132–2143 (2010).
Landskron, J. & Taskén, K. CD147 in regulatory T cells. Cell. Immunol. 282, 17–20 (2013).
Tehranchi, R. et al. Granulocyte-colony-stimulating factor inhibits spontaneous cytochrome c release and mitochondria-dependent apoptosis of myelodysplastic syndrome hematopoietic progenitors. Blood 101, 1080–1086 (2003).
Gloeckner, C.J., Boldt, K., Schumacher, A., Roepman, R. & Ueffing, M. A novel tandem-affinity purification strategy for the efficient isolation and characterization of native protein complexes. Proteomics 7, 4228–4234 (2007).
Le Floch, R. et al. CD147 subunit of lactate–H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc. Natl. Acad. Sci. USA 108, 16663–16668 (2011).
Birsoy, K. et al. MCT1-mediated transport of a toxic molecule is an effective strategy for targeting glycolytic tumors. Nat. Genet. 45, 104–108 (2013).
Lo, Y.H., Ho, P.C. & Wang, S.C. Epidermal growth factor receptor protects proliferating cell nuclear antigen from cullin 4A protein-mediated proteolysis. J. Biol. Chem. 287, 27148–27157 (2012).
Bassermann, F. et al. The Cdc14B–Cdh1–Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell 134, 256–267 (2008).
Fernández-Sáiz, V. et al. SCFFbxo9 and CK2 direct the cellular response to growth factor withdrawal via Tel2/Tti1 degradation and promote survival in multiple myeloma. Nat. Cell Biol. 15, 72–81 (2013).
Baumann, U. et al. Disruption of the PRKCD–FBXO25–HAX-1 axis attenuates the apoptotic response and drives lymphomagenesis. Nat. Med. 20, 1401–1409 (2014).
Schmid, B. et al. Loss of ALS-associated TDP-43 in zebrafish causes muscle degeneration, vascular dysfunction and reduced motor neuron axon outgrowth. Proc. Natl. Acad. Sci. USA 110, 4986–4991 (2013).
Kennedy, B.N. et al. Zebrafish rx3 and mab21l2 are required during eye morphogenesis. Dev. Biol. 270, 336–349 (2004).
Acknowledgements
We thank M. Buschbeck (Institute of Predictive and Personalized Medicine of Cancer, Barcelona) for the SKK-1 and SKM-1 cell lines, X. Bustelo (Centro de Investigacion del Cancer, Salamanca) for a EGFP–CD147 expression plasmid, Y. Cang (Life Science Institute, Zheijang University) for a CRBN expression construct, B. Ebert (Dana-Farber Cancer Institute) for an IKZF3 expression construct, A. Halestrap (School of Biochemistry, University of Bristol) for MCT1 expression constructs, H. Handa (Tokyo Institute of Technology, Japan) for an aliquot of mouse monoclonal CRBN-specific antibody, K. Kadomatsu (Department of Molecular Biology, Nagoya University) for CD147 expression constructs, W. Kaelin (Dana-Farber Cancer Institute) for CRISPR-based HEK293FT CBRN−/− cells and two clones of MM1.S CBRN−/− cells (T11, T21), D. Sabatini (Whitehead Institute) for a MCT1 and an shMCT1 construct, K. Stewart (Mayo Clinic) for a shCRBN plasmid, N. Thomä (Friedrich Miescher Institute for Biomedical Research, Basel) for a MEIS2 expression construct and purified CRL4CRBN protein, K. Tohyama (Kawasaki Medical School, Okayama) for the del(5q) MDS cell line MDSL, N. Thomä and E. Fisher for suggestions, R. Rojas for fish care and B. Nuscher for help with gel filtration chromatography. This work was supported by the Helmholtz cross-program topic 'Metabolic Dysfunction' (B.S.), a fellowship of the TU Munich (KKF # B10-13; to R.E.), grants from the José Carreras Leukemia Foundation (DJCLS R 14/16; to K.S.G.), the German Research Foundation (FOR2033 GO 713/2-1 and SFB 1243 (K.S.G.), SFB 824 (U.K.), KE 222/7-1 (U.K.), BA 2851/4-1 (F.B.) and SFB 1243 (F.B.)), the German Cancer Aid (#111051 (M.H.), #111305 (U.K.) and #111430 (F.B.)) and the Wilhelm Sander Stiftung (#2012.096.1; to F.B.). An application for a patent has been filed at the European Patent Office.
Author information
Authors and Affiliations
Contributions
R.E., M.H., V.F.-S. and F.B. conceived and designed the research; R.E., M.H. and V.F.-S. performed most of the experiments with crucial help from B.-S.T. and A.-M.K.; U.P., U.G. and K.S.G. provided MDS samples; C.L., S.K. and H.E. provided MM samples; K.S.G. and A.-K.G. performed FACS analyses; J.S. helped with the MM xenotransplant model; L.J. helped with the PET analysis; M.R. performed immunohistochemical analyses of tumors; F.v.B., B.S. and C.H. performed zebrafish experiments; S.L. and B.K. performed mass spectrometry; R.E., M.H., V.F.-S., F.v.B., A.-K.G., B.-S.T., S.L., U.K., C.P., B.S., C.H., K.S.G., B.K. and F.B. analyzed results; and F.B. coordinated this work and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
F.B. and K.S.G. received honoraria and research funding from Celgene.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 and Supplementary Table 2 (PDF 5435 kb)
Supplementary Table 1
Strep-Flag-Tandem affinity purified-CRBN-associated proteins identified by mass spectrometry analysis. (XLSX 24 kb)
Rights and permissions
About this article
Cite this article
Eichner, R., Heider, M., Fernández-Sáiz, V. et al. Immunomodulatory drugs disrupt the cereblon–CD147–MCT1 axis to exert antitumor activity and teratogenicity. Nat Med 22, 735–743 (2016). https://doi.org/10.1038/nm.4128
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4128
This article is cited by
-
The CB1 receptor interacts with cereblon and drives cereblon deficiency-associated memory shortfalls
EMBO Molecular Medicine (2024)
-
The role of lactate in cardiovascular diseases
Cell Communication and Signaling (2023)
-
Multiple myeloma with t(11;14): impact of novel agents on outcome
Blood Cancer Journal (2023)
-
Tumor lactic acid: a potential target for cancer therapy
Archives of Pharmacal Research (2023)
-
Genome-scale functional genomics identify genes preferentially essential for multiple myeloma cells compared to other neoplasias
Nature Cancer (2023)