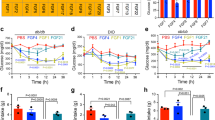
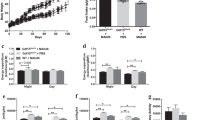
Abstract
Type 2 diabetes (T2D) is among the most common and costly disorders worldwide1. The goal of current medical management for T2D is to transiently ameliorate hyperglycemia through daily dosing of one or more antidiabetic drugs. Hypoglycemia and weight gain are common side effects of therapy, and sustained disease remission is not obtainable with nonsurgical approaches. On the basis of the potent glucose-lowering response elicited by activation of brain fibroblast growth factor (FGF) receptors2,3,4, we explored the antidiabetic efficacy of centrally administered FGF1, which, unlike other FGF peptides, activates all FGF receptor subtypes5. We report that a single intracerebroventricular injection of FGF1 at a dose one-tenth of that needed for antidiabetic efficacy following peripheral injection induces sustained diabetes remission in both mouse and rat models of T2D. This antidiabetic effect is not secondary to weight loss, does not increase the risk of hypoglycemia, and involves a novel and incompletely understood mechanism for increasing glucose clearance from the bloodstream. We conclude that the brain has an inherent potential to induce diabetes remission and that brain FGF receptors are potential pharmacological targets for achieving this goal.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
International Diabetes Federation. IDF Diabetes Atlas 7th edn. (International Diabetes Federation, Brussels, Belgium, 2015).
Morton, G.J. et al. FGF19 action in the brain induces insulin-independent glucose lowering. J. Clin. Invest. 123, 4799–4808 (2013).
Marcelin, G. et al. Central action of FGF19 reduces hypothalamic AgRP/NPY neuron activity and improves glucose metabolism. Mol. Metab. 3, 19–28 (2014).
Ryan, K.K. et al. Fibroblast growth factor 19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats. Endocrinology 154, 9–15 (2013).
Ornitz, D.M. & Itoh, N. The fibroblast growth factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 4, 215–266 (2015).
Schwartz, M.W. et al. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature 503, 59–66 (2013).
Grayson, B.E., Seeley, R.J. & Sandoval, D.A. Wired on sugar: the role of the CNS in the regulation of glucose homeostasis. Nat. Rev. Neurosci. 14, 24–37 (2013).
Fu, L. et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 145, 2594–2603 (2004).
Xu, J. et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models—association with liver and adipose tissue effects. Am. J. Physiol. Endocrinol. Metab. 297, E1105–E1114 (2009).
Suh, J.M. et al. Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer. Nature 513, 436–439 (2014).
Ito, J. et al. Astrocytes produce and secrete FGF1, which promotes the production of apoE–HDL in a manner of autocrine action. J. Lipid Res. 46, 679–686 (2005).
Oomura, Y. et al. A new brain glucosensor and its physiological significance. Am. J. Clin. Nutr. 55 (suppl. 1), 278S–282S (1992).
Suzuki, S. et al. Feeding suppression by fibroblast growth factor 1 is accompanied by selective induction of heat-shock protein 27 in hypothalamic astrocytes. Eur. J. Neurosci. 13, 2299–2308 (2001).
Lou, G. et al. Intranasal administration of TAT–haFGF14–154 attenuates disease progression in a mouse model of Alzheimer's disease. Neuroscience 223, 225–237 (2012).
Cheng, X. et al. Acidic fibroblast growth factor delivered intranasally induces neurogenesis and angiogenesis in rats after ischemic stroke. Neurol. Res. 33, 675–680 (2011).
Jonker, J.W. et al. A PPAR-γ–FGF1 axis is required for adaptive adipose remodeling and metabolic homeostasis. Nature 485, 391–394 (2012).
Perry, R.J. et al. FGF1 and FGF19 reverse diabetes by suppression of the hypothalamic–pituitary–adrenal axis. Nat. Commun. 6, 6980 (2015).
Best, J.D., Taborsky, G.J. Jr., Halter, J.B. & Porte, D. Jr. Glucose disposal is not proportional to plasma glucose level in man. Diabetes 30, 847–850 (1981).
Kahn, S.E. et al. The contribution of insulin-dependent and insulin-independent glucose uptake to intravenous glucose tolerance in healthy human subjects. Diabetes 43, 587–592 (1994).
Gresl, T.A. et al. Dietary restriction and glucose regulation in aging rhesus monkeys: a follow-up report at 8.5 years. Am. J. Physiol. Endocrinol. Metab. 281, E757–E765 (2001).
Ader, M., Pacini, G., Yang, Y.J. & Bergman, R.N. Importance of glucose per se to intravenous glucose tolerance. Comparison of the minimal-model prediction with direct measurements. Diabetes 34, 1092–1103 (1985).
Alonso, L.C. et al. Simultaneous measurement of insulin sensitivity, insulin secretion, and the disposition index in conscious unhandled mice. Obesity (Silver Spring) 20, 1403–1412 (2012).
Rojas, J.M. et al. Glucose intolerance induced by blockade of central FGF receptors is linked to an acute stress response. Mol. Metab. 4, 561–568 (2015).
Stefanovski, D. et al. Estimating hepatic glucokinase activity using a simple model of lactate kinetics. Diabetes Care 35, 1015–1020 (2012).
Davis, M.A., Williams, P.E. & Cherrington, A.D. Net hepatic lactate balance following mixed meal feeding in the 4-d fasted conscious dog. Metabolism 36, 856–862 (1987).
King, A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 166, 877–894 (2012).
Porte, D. Jr. Banting lecture 1990. Beta cells in type 2 diabetes mellitus. Diabetes 40, 166–180 (1991).
Schäffer, L. et al. A novel high-affinity peptide antagonist to the insulin receptor. Biochem. Biophys. Res. Commun. 376, 380–383 (2008).
Lu, M. et al. Insulin regulates liver metabolism in vivo in the absence of hepatic AKT and FOXO1. Nat. Med. 18, 388–395 (2012).
O-Sullivan, I. et al. FOXO1 integrates direct and indirect effects of insulin on hepatic glucose production and glucose utilization. Nat. Commun. 6, 7079 (2015).
Titchenell, P.M., Chu, Q., Monks, B.R. & Birnbaum, M.J. Hepatic insulin signaling is dispensable for suppression of glucose output by insulin in vivo. Nat. Commun. 6, 7078 (2015).
Orellana, J.A. et al. Glucose increases intracellular free Ca2+ in tanycytes via ATP released through connexin 43 hemichannels. Glia 60, 53–68 (2012).
Robins, S.C. et al. α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat. Commun. 4, 2049 (2013).
Stetler, R.A., Gao, Y., Signore, A.P., Cao, G. & Chen, J. HSP27: mechanisms of cellular protection against neuronal injury. Curr. Mol. Med. 9, 863–872 (2009).
Mirzadeh, Z., Doetsch, F., Sawamoto, K., Wichterle, H. & Alvarez-Buylla, A. The subventricular zone en-face: whole-mount staining and ependymal flow. J. Vis. Exp. 39, 1938 (2010).
Fischer, A., Sananbenesi, F., Wang, X., Dobbin, M. & Tsai, L.H. Recovery of learning and memory is associated with chromatin remodeling. Nature 447, 178–182 (2007).
Pinto, J.G., Jones, D.G., Williams, C.K. & Murphy, K.M. Characterizing synaptic protein development in human visual cortex enables alignment of synaptic age with rat visual cortex. Front. Neural Circuits 9, 3 (2015).
Moore, M.C., Coate, K.C., Winnick, J.J., An, Z. & Cherrington, A.D. Regulation of hepatic glucose uptake and storage in vivo. Adv. Nutr. 3, 286–294 (2012).
Elizondo-Vega, R. et al. The role of tanycytes in hypothalamic glucosensing. J. Cell. Mol. Med. 19, 1471–1482 (2015).
Bolborea, M. & Dale, N. Hypothalamic tanycytes: potential roles in the control of feeding and energy balance. Trends Neurosci. 36, 91–100 (2013).
National Research Council. Guide for the Care and Use of Laboratory Animals 8th edn. (The National Academies Press, 2011).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates (Academic Press, San Diego, California, USA, 1998).
Franklin, K.B.J. & Paxinos, G. The Mouse Brain in Stereotaxic Coordinates (Academic Press, San Diego, California, USA, 1997).
Ayala, J.E., Bracy, D.P., McGuinness, O.P. & Wasserman, D.H. Considerations in the design of hyperinsulinemic–euglycemic clamps in the conscious mouse. Diabetes 55, 390–397 (2006).
Otero, Y.F. et al. Enhanced glucose transport but not phosphorylation capacity ameliorates lipopolysaccharide-induced impairments in insulin-stimulated muscle glucose uptake. Shock http://dx.doi.org/10.1097/SHK.0000000000000550 (2015).
Acknowledgements
The authors are grateful to the Vanderbilt University Mouse Metabolic Phenotyping Center (DK059637) for the performance of the basal glucose turnover, FSIGT and [2-14C]DG studies, the Nutrition Obesity Research Center (DK035816), the Diabetes Research Center (DK017047) at the University of Washington, and the technical assistance provided by T. Meek, V. Damian, L. Nguyen, T. Harvey, and J. Brown (all at University of Washington) and by D. Bracy and A. Locke (both at Vanderbilt University). We gratefully acknowledge L. Schäffer (Novo Nordisk) for providing the insulin receptor antagonist (S961). This work was supported by the US National Institute of Diabetes and Digestive and Kidney Diseases (grant nos. DK083042 (M.W.S.), DK090320 (M.W.S.), DK101997 (M.W.S.), DK089056 (G.J.M.), DK007742 (J.M.S.), DK104461 (J.M.S.), DK007247 (J.M.R.), DK103375 (J.M.R.), DK27619 (R.N.B.), and DK29867 (R.N.B.)), the Department of Veterans Affairs Merit Review Program (T.G.U.), and by funding supplied by Novo Nordisk (M.W.S.).
Author information
Authors and Affiliations
Contributions
J.M.S., J.M.R., L.L., D.H.W., G.J.M., and M.W.S., designed, funded, and supervised the research; J.M.S., J.M.R., M.D.D., Z.M., and M.E.M. performed the research; T.G.U. generated the LIRFKO mice; J.M.S., J.M.R., K.J.K., D.S., M.D.D., Z.M., H.T.N., R.N.B., L.L., D.H.W., G.J.M., and M.W.S. analyzed the data; and J.M.S., J.M.R., and M.W.S. wrote the manuscript. M.W.S. has final responsibility for the hypothesis, the study design, the data analysis, the interpretation and conclusions, and the final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Funding for these studies was provided to M.W.S. in part by Novo Nordisk.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 590 kb)
Rights and permissions
About this article
Cite this article
Scarlett, J., Rojas, J., Matsen, M. et al. Central injection of fibroblast growth factor 1 induces sustained remission of diabetic hyperglycemia in rodents. Nat Med 22, 800–806 (2016). https://doi.org/10.1038/nm.4101
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4101
This article is cited by
-
Mediation effect of serum zinc on insulin secretion inhibited by methyl tert-butyl ether in gas station workers
Environmental Science and Pollution Research (2024)
-
The ventromedial hypothalamic nucleus: watchdog of whole-body glucose homeostasis
Cell & Bioscience (2022)
-
Glial cells as integrators of peripheral and central signals in the regulation of energy homeostasis
Nature Metabolism (2022)
-
Metabolic Messengers: fibroblast growth factor 1
Nature Metabolism (2022)
-
Stimulation of the hepatoportal nerve plexus with focused ultrasound restores glucose homoeostasis in diabetic mice, rats and swine
Nature Biomedical Engineering (2022)