Abstract
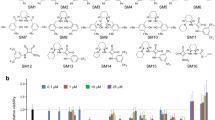
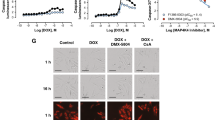
Doxorubicin is an anthracycline chemotherapy agent effective in treating a wide range of malignancies, but it causes a dose-related cardiotoxicity that can lead to heart failure in a subset of patients. At present, it is not possible to predict which patients will be affected by doxorubicin-induced cardiotoxicity (DIC). Here we demonstrate that patient-specific human induced pluripotent stem cell–derived cardiomyocytes (hiPSC-CMs) can recapitulate the predilection to DIC of individual patients at the cellular level. hiPSC-CMs derived from individuals with breast cancer who experienced DIC were consistently more sensitive to doxorubicin toxicity than hiPSC-CMs from patients who did not experience DIC, with decreased cell viability, impaired mitochondrial and metabolic function, impaired calcium handling, decreased antioxidant pathway activity, and increased reactive oxygen species production. Taken together, our data indicate that hiPSC-CMs are a suitable platform to identify and characterize the genetic basis and molecular mechanisms of DIC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lipshultz, S.E., Franco, V.I., Miller, T.L., Colan, S.D. & Sallan, S.E. Cardiovascular disease in adult survivors of childhood cancer. Annu. Rev. Med. 66, 161–176 (2015).
Giordano, S.H., Lin, Y.-L., Kuo, Y.F., Hortobagyi, G.N. & Goodwin, J.S. Decline in the use of anthracyclines for breast cancer. J. Clin. Oncol. 30, 2232–2239 (2012).
Lefrak, E.A., Pitha, J., Rosenheim, S. & Gottlieb, J.A. A clinicopathologic analysis of Adriamycin cardiotoxicity. Cancer 32, 302–314 (1973).
Von Hoff, D.D. et al. Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med. 91, 710–717 (1979).
Swain, S.M., Whaley, F.S. & Ewer, M.S. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 97, 2869–2879 (2003).
Kremer, L.C.M., van der Pal, H.J.H., Offringa, M., Van Dalen, E.C. & Voûte, P.A. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Ann. Oncol. 13, 819–829 (2002).
Shakir, D.K. & Rasul, K.I. Chemotherapy-induced cardiomyopathy: pathogenesis, monitoring, and management. J. Clin. Med. Res. 1, 8–12 (2009).
Bernstein, D. & Burridge, P. Patient-specific pluripotent stem cells in doxorubicin cardiotoxicity: a new window into personalized medicine. Prog. Pediatr. Cardiol. 37, 23–27 (2014).
Lipshultz, S.E., Cochran, T.R., Franco, V.I. & Miller, T.L. Treatment-related cardiotoxicity in survivors of childhood cancer. Nat. Rev. Clin. Oncol. 10, 697–710 (2013).
Granger, C.B. Prediction and prevention of chemotherapy-induced cardiomyopathy: can it be done? Circulation 114, 2432–2433 (2006).
Deavall, D.G., Martin, E.A., Horner, J.M. & Roberts, R. Drug-induced oxidative stress and toxicity. J. Toxicol. 2012, 645460 (2012).
Zhang, S. et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 18, 1639–1642 (2012).
Khiati, S. et al. Mitochondrial topoisomerase I (Top1mt) is a novel limiting factor of doxorubicin cardiotoxicity. Clin. Cancer Res. 20, 4873–4881 (2014).
Hanna, A.D., Lam, A., Tham, S., Dulhunty, A.F. & Beard, N.A. Adverse effects of doxorubicin and its metabolic product on cardiac RyR2 and SERCA2A. Mol. Pharmacol. 86, 438–449 (2014).
Goormaghtigh, E., Brasseur, R., Huart, P. & Ruysschaert, J.M. Study of the Adriamycin–cardiolipin complex structure using attenuated total-reflection infrared spectroscopy. Biochemistry 26, 1789–1794 (1987).
Ichikawa, Y. et al. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Invest. 124, 617–630 (2014).
Holmberg, S.R. & Williams, A.J. Patterns of interaction between anthraquinone drugs and the calcium-release channel from cardiac sarcoplasmic reticulum. Circ. Res. 67, 272–283 (1990).
Burridge, P.W. et al. Modeling cardiovascular diseases with patient-specific human pluripotent stem cell–derived cardiomyocytes. Methods Mol. Biol. 1353, 119–130 (2016).
Cahan, P. & Daley, G.Q. Origins and implications of pluripotent stem cell variability and heterogeneity. Nat. Rev. Mol. Cell Biol. 14, 357–368 (2013).
Peterson, S.E. & Loring, J.F. Genomic instability in pluripotent stem cells: implications for clinical applications. J. Biol. Chem. 289, 4578–4584 (2014).
Burridge, P.W. et al. Chemically defined generation of human cardiomyocytes. Nat. Methods 11, 855–860 (2014).
Burridge, P.W., Holmström, A. & Wu, J.C. Chemically defined culture and cardiomyocyte differentiation of human pluripotent stem cells. Curr. Protoc. Hum. Genet. 87, 21.3 (2015).
Rana, P., Anson, B., Engle, S. & Will, Y. Characterization of human induced pluripotent stem cell–derived cardiomyocytes: bioenergetics and utilization in safety screening. Toxicol. Sci. 130, 117–131 (2012).
Yang, X. et al. Triiodo-L-thyronine promotes the maturation of human cardiomyocytes derived from induced pluripotent stem cells. J. Mol. Cell. Cardiol. 72, 296–304 (2014).
Robert, J. et al. Comparative pharmacokinetics and metabolism of doxorubicin and epirubicin in patients with metastatic breast cancer. Cancer Treat. Rep. 69, 633–640 (1985).
Bramwell, V.H.C. et al. Safety and efficacy of the multidrug-resistance inhibitor biricodar (VX-710) with concurrent doxorubicin in patients with anthracycline-resistant advanced soft tissue sarcoma. Clin. Cancer Res. 8, 383–393 (2002).
Berdichevski, A. et al. TVP1022 protects neonatal rat ventricular myocytes against doxorubicin-induced functional derangements. J. Pharmacol. Exp. Ther. 332, 413–420 (2010).
Ito, H. et al. Doxorubicin selectively inhibits muscle gene expression in cardiac muscle cells in vivo and in vitro. Proc. Natl. Acad. Sci. USA 87, 4275–4279 (1990).
Ruan, Y. et al. SIRT1 suppresses doxorubicin-induced cardiotoxicity by regulating the oxidative stress and p38MAPK pathways. Cell. Physiol. Biochem. 35, 1116–1124 (2015).
Lim, C.C. et al. Anthracyclines induce calpain-dependent titin proteolysis and necrosis in cardiomyocytes. J. Biol. Chem. 279, 8290–8299 (2004).
Chen, B. et al. Disruption of a GATA4–ANKRD1 signaling axis in cardiomyocytes leads to sarcomere disarray: implications for anthracycline cardiomyopathy. PLoS One 7, e35743 (2012).
Doroshow, J.H. Effect of anthracycline antibiotics on oxygen radical formation in rat heart. Cancer Res. 43, 460–472 (1983).
Shi, Y., Moon, M., Dawood, S., McManus, B. & Liu, P.P. Mechanisms and management of doxorubicin cardiotoxicity. Herz 36, 296–305 (2011).
Xu, X., Persson, H.L. & Richardson, D.R. Molecular pharmacology of the interaction of anthracyclines with iron. Mol. Pharmacol. 68, 261–271 (2005).
Venditti, P., Balestrieri, M., De Leo, T. & Di Meo, S. Free radical involvement in doxorubicin-induced electrophysiological alterations in rat papillary muscle fibers. Cardiovasc. Res. 38, 695–702 (1998).
Swain, S.M. et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J. Clin. Oncol. 15, 1318–1332 (1997).
Deng, S. et al. The catalytic topoisomerase II inhibitor dexrazoxane induces DNA breaks, ATF3, and the DNA damage response in cancer cells. Br. J. Pharmacol. 172, 2246–2257 (2015).
Farshid, A.A. et al. Effects of histidine and N-acetylcysteine on doxorubicin-induced cardiomyopathy in rats. Cardiovasc. Toxicol. 14, 153–161 (2014).
Engreitz, J.M., Daigle, B.J. Jr., Marshall, J.J. & Altman, R.B. Independent component analysis: mining microarray data for fundamental human gene expression modules. J. Biomed. Inform. 43, 932–944 (2010).
Thorn, C.F. et al. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet. Genomics 21, 440–446 (2011).
Hussner, J. et al. Regulation of interferon-inducible proteins by doxorubicin via interferon-γ–Janus tyrosine kinase–signal transducer and activator of transcription signaling in tumor cells. Mol. Pharmacol. 81, 679–688 (2012).
Zhu, W., Zhang, W., Shou, W. & Field, L.J. p53 inhibition exacerbates late-stage anthracycline cardiotoxicity. Cardiovasc. Res. 103, 81–89 (2014).
Arts-de Jong, M., Maas, A.H.E.M., Massuger, L.F., Hoogerbrugge, N. & de Hullu, J.A. BRCA1/2 mutation carriers are potentially at higher cardiovascular risk. Crit. Rev. Oncol. Hematol. 91, 159–171 (2014).
Doroshow, J.H., Locker, G.Y. & Myers, C.E. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J. Clin. Invest. 65, 128–135 (1980).
Torti, S.V., Akimoto, H., Lin, K., Billingham, M.E. & Torti, F.M. Selective inhibition of muscle gene expression by oxidative stress in cardiac cells. J. Mol. Cell. Cardiol. 30, 1173–1180 (1998).
Naidu, S.R., Love, I.M., Imbalzano, A.N., Grossman, S.R. & Androphy, E.J. The SWI/SNF chromatin remodeling subunit BRG1 is a critical regulator of p53 necessary for proliferation of malignant cells. Oncogene 28, 2492–2501 (2009).
Visscher, H. et al. CPNDS Consortium. Validation of variants in SLC28A3 and UGT1A6 as genetic markers predictive of anthracycline-induced cardiotoxicity in children. Pediatr. Blood Cancer 60, 1375–1381 (2013).
Lebrecht, D., Kokkori, A., Ketelsen, U.-P., Setzer, B. & Walker, U.A. Tissue-specific mtDNA lesions and radical-associated mitochondrial dysfunction in human hearts exposed to doxorubicin. J. Pathol. 207, 436–444 (2005).
Octavia, Y. et al. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J. Mol. Cell. Cardiol. 52, 1213–1225 (2012).
Denning, C. et al. Cardiomyocytes from human pluripotent stem cells: from laboratory curiosity to industrial biomedical platform. Biochim. Biophys. Acta http://dx.doi:10.1016/j.bbamcr.2015.10.014 (2015).
Zhu, R. et al. Physical developmental cues for the maturation of human pluripotent stem cell–derived cardiomyocytes. Stem Cell Res. Ther. 5, 117 (2014).
David, R. & Franz, W.M. From pluripotency to distinct cardiomyocyte subtypes. Physiology (Bethesda) 27, 119–129 (2012).
Mathur, A. et al. Human iPSC-based cardiac microphysiological system for drug-screening applications. Sci. Rep. 5, 8883 (2015).
Mercola, M., Colas, A. & Willems, E. Induced pluripotent stem cells in cardiovascular drug discovery. Circ. Res. 112, 534–548 (2013).
Brown, S.-A., Sandhu, N. & Herrmann, J. Systems biology approaches to adverse drug effects: the example of cardio-oncology. Nat. Rev. Clin. Oncol. 12, 718–731 (2015).
Aminkeng, F. et al. A coding variant in RARG confers susceptibility to anthracycline-induced cardiotoxicity in childhood cancer. Nat. Genet. 47, 1079–1084 (2015).
Vejpongsa, P. & Yeh, E.T.H. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J. Am. Coll. Cardiol. 64, 938–945 (2014).
Kim, H.D., Kim, C.H., Rah, B.J., Chung, H.I. & Shim, T.S. Quantitative study on the relation between structural and functional properties of the hearts from three different mammals. Anat. Rec. 238, 199–206 (1994).
Hattori, F. et al. Nongenetic method for purifying stem cell–derived cardiomyocytes. Nat. Methods 7, 61–66 (2010).
Melkoumian, Z. et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat. Biotechnol. 28, 606–610 (2010).
Chen, G. et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 8, 424–429 (2011).
Fusaki, N., Ban, H., Nishiyama, A., Saeki, K. & Hasegawa, M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc. Jpn. Acad., Ser. B, Phys. Biol. Sci. 85, 348–362 (2009).
Mali, P. et al. Butyrate greatly enhances derivation of human induced pluripotent stem cells by promoting epigenetic remodeling and the expression of pluripotency-associated genes. Stem Cells 28, 713–720 (2010).
Thomson, J.A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998).
Maddah, M. et al. A non-invasive platform for functional characterization of stem cell–derived cardiomyocytes with applications in cardiotoxicity testing. Stem Cell Rep. 4, 621–631 (2015).
Huang, X. & Darzynkiewicz, Z. Cytometric assessment of histone H2AX phosphorylation: a reporter of DNA damage. Methods Mol. Biol. 314, 73–80 (2006).
Mukhopadhyay, P. et al. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat. Protoc. 2, 2295–2301 (2007).
Irizarry, R.A. et al. Exploration, normalization, and summaries of high-density oligonucleotide array probe level data. Biostatistics 4, 249–264 (2003).
Bolstad, B.M., Irizarry, R.A., Astrand, M. & Speed, T.P. A comparison of normalization methods for high-density oligonucleotide array data based on variance and bias. Bioinformatics 19, 185–193 (2003).
Kim, D. et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions, and gene fusions. Genome Biol. 14, R36 (2013).
Trapnell, C. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578 (2012).
Li, H. et al. The sequence alignment–map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Cingolani, P. et al. A program for annotating and predicting the effects of single-nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118;iso-2;iso-3. Fly (Austin) 6, 80–92 (2012).
Robinson, J.T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
Hyvärinen, A. & Oja, E. Independent component analysis: algorithms and applications. Neural Netw. 13, 411–430 (2000).
Lee, S.-I. & Batzoglou, S. Application of independent component analysis to microarrays. Genome Biol. 4, R76 (2003).
Alexa, A., Rahnenführer, J. & Lengauer, T. Improved scoring of functional groups from gene expression data by de-correlating GO graph structure. Bioinformatics 22, 1600–1607 (2006).
Gerstein, M.B. et al. Architecture of the human regulatory network derived from ENCODE data. Nature 489, 91–100 (2012).
Barrett, T. et al. NCBI GEO: archive for functional genomics data sets—update. Nucleic Acids Res. 41, D991–D995 (2013).
Acknowledgements
We thank J. Odegaard for analysis of teratoma slides. This work was supported by the US National Institutes of Health (NIH) grants K99/R00 HL121177 (P.W.B.), R21 HL123655 (D.B.), R01 LM05652 (R.B.A.), R01 GM102365 (R.B.A.), R24 GM61374 (R.B.A.), R01 HL123968 (J.C.W.), R01 HL126527 (J.C.W.), R01 HL128170 (J.C.W.), R01 HL130020 (J.C.W.), R01 AR063963 (H.M.B.), R01 AG020961 (H.M.B.), R21 AG04481501 (H.M.B.), and R01 NS089533 (H.M.B.); the American Heart Association (AHA) grants AHA 14BGIA20480329 (P.W.B.), AHA 13POST14480004 (A.C.C.), and AHA 13EIA14420025 (J.C.W.); a Dixon Translational Research Grant Young Investigator Award (P.W.B.), the California Institute of Regenerative Medicine (CIRM) awards IT1-06596 (J.C.W.), TR3-05501 (H.M.B.) and RB5-07469 (H.M.B.); the Muscular Dystrophy Association grant 4320 (H.M.B.); the Baxter Foundation (H.M.B.); and a Burroughs Wellcome Fund Innovation in Regulatory Science Award (J.C.W.).
Author information
Authors and Affiliations
Contributions
P.W.B. performed project planning, experimental design, hiPSC reprogramming, cell culture, characterizations, differentiation, cardiotoxicity analysis, flow cytometry, data analysis, and wrote the manuscript; Y.F.L. performed computational analyses of microarray and RNA-seq data, gene enrichment analysis, and wrote part of the manuscript; E.M. and A.H. performed cell culture; E.M. and A.S. performed immunohistochemistry; H.W. performed Ca2+ imaging; P.W.B., S.-G.O., A.C.C., M.J.C., and A.D.E. performed Seahorse analysis; S.-G.O. performed mitochondrial analysis; J.W.K., M.L.T., and R.M.W. recruited patients; H.M.B., D.B., R.B.A., and J.C.W. helped in manuscript preparation; J.C.W. and P.W.B. provided conceptual design of the study and funding support.
Corresponding authors
Ethics declarations
Competing interests
P.W.B. is a shareholder in Stem Cell Theranostics, and J.C.W. is a cofounder and shareholder in Stem Cell Theranostics.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9, Supplementary Tables 1–6 and Supplementary Note (PDF 11860 kb)
Rights and permissions
About this article
Cite this article
Burridge, P., Li, Y., Matsa, E. et al. Human induced pluripotent stem cell–derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med 22, 547–556 (2016). https://doi.org/10.1038/nm.4087
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4087
This article is cited by
-
Biosafe cerium oxide nanozymes protect human pluripotent stem cells and cardiomyocytes from oxidative stress
Journal of Nanobiotechnology (2024)
-
In situ monolayer patch clamp of acutely stimulated human iPSC-derived cardiomyocytes promotes consistent electrophysiological responses to SK channel inhibition
Scientific Reports (2024)
-
A review of the pathophysiological mechanisms of doxorubicin-induced cardiotoxicity and aging
npj Aging (2024)
-
An In Silico Platform to Predict Cardiotoxicity Risk of Anti-tumor Drug Combination with hiPSC-CMs Based In Vitro Study
Pharmaceutical Research (2024)
-
Modeling drug-induced mitochondrial toxicity with human primary cardiomyocytes
Science China Life Sciences (2024)