Abstract
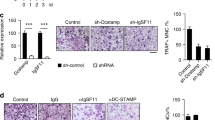
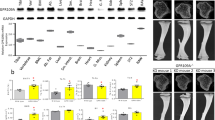
Tumor necrosis factor (TNF) superfamily member 11 (TNFSF11, also known as RANKL) regulates multiple physiological or pathological functions, including osteoclast differentiation and osteoporosis. TNFRSF11A (also called RANK) is considered to be the sole receptor for RANKL. Herein we report that leucine-rich repeat-containing G-protein-coupled receptor 4 (LGR4, also called GPR48) is another receptor for RANKL. LGR4 competes with RANK to bind RANKL and suppresses canonical RANK signaling during osteoclast differentiation. RANKL binding to LGR4 activates the Gαq and GSK3-β signaling pathway, an action that suppresses the expression and activity of nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent 1 (NFATC1) during osteoclastogenesis. Both whole-body (Lgr4−/−) and monocyte conditional knockout mice of Lgr4 (Lgr4 CKO) exhibit osteoclast hyperactivation (including elevation of osteoclast number, surface area, and size) and increased bone erosion. The soluble LGR4 extracellular domain (ECD) binds RANKL and inhibits osteoclast differentiation in vivo. Moreover, LGR4-ECD therapeutically abrogated RANKL-induced bone loss in three mouse models of osteoporosis. Therefore, LGR4 acts as a second RANKL receptor that negatively regulates osteoclast differentiation and bone resorption.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kong, Y.Y. et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397, 315–323 (1999).
Hanada, R., Hanada, T., Sigl, V., Schramek, D. & Penninger, J.M. RANKL/RANK-beyond bones. J. Mol. Med. 89, 647–656 (2011).
Fata, J.E. et al. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103, 41–50 (2000).
Jones, D.H. et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 440, 692–696 (2006).
Gonzalez-Suarez, E. et al. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature 468, 103–107 (2010).
Tan, W. et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 470, 548–553 (2011).
Schramek, D. et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature 468, 98–102 (2010).
Kiechl, S. et al. Blockade of receptor activator of nuclear factor-κB (RANKL) signaling improves hepatic insulin resistance and prevents development of diabetes mellitus. Nat. Med. 19, 358–363 (2013).
Hanada, R. et al. Central control of fever and female body temperature by RANKL/RANK. Nature 462, 505–509 (2009).
Simonet, W.S. et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89, 309–319 (1997).
Lacey, D.L. et al. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat. Rev. Drug Discov. 11, 401–419 (2012).
Weng, J. et al. Deletion of G protein-coupled receptor 48 leads to ocular anterior segment dysgenesis (ASD) through down-regulation of Pitx2. Proc. Natl. Acad. Sci. USA 105, 6081–6086 (2008).
Luo, J. et al. Regulation of bone formation and remodeling by G-protein-coupled receptor 48. Development 136, 2747–2756 (2009).
Carmon, K.S., Gong, X., Lin, Q., Thomas, A. & Liu, Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc. Natl. Acad. Sci. USA 108, 11452–11457 (2011).
de Lau, W. et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476, 293–297 (2011).
Styrkarsdottir, U. et al. Nonsense mutation in the LGR4 gene is associated with several human diseases and other traits. Nature 497, 517–520 (2013).
Abe, E. et al. TSH is a negative regulator of skeletal remodeling. Cell 115, 151–162 (2003).
Sun, L. et al. FSH directly regulates bone mass. Cell 125, 247–260 (2006).
Du, B. et al. Lgr4/Gpr48 negatively regulates TLR2/4-associated pattern recognition and innate immunity by targeting CD14 expression. J. Biol. Chem. 288, 15131–15141 (2013).
Wang, Y. et al. Lgr4 regulates mammary gland development and stem cell activity through the pluripotency transcription factor Sox2. Stem Cells 31, 1921–1931 (2013).
Wang, J. et al. Ablation of LGR4 promotes energy expenditure by driving white-to-brown fat switch. Nat. Cell Biol. 15, 1455–1463 (2013).
Gao, Y. et al. Up-regulation of GPR48 induced by down-regulation of p27Kip1 enhances carcinoma cell invasiveness and metastasis. Cancer Res. 66, 11623–11631 (2006).
Wu, J. et al. GPR48, a poor prognostic factor, promotes tumor metastasis and activates β-catenin/TCF signaling in colorectal cancer. Carcinogenesis 34, 2861–2869 (2013).
Joshi, P.A. et al. RANK signaling amplifies WNT-responsive mammary progenitors through R-SPONDIN1. Stem Cell Reports 5, 31–44 (2015).
Wang, D. et al. Structural basis for R-spondin recognition by LGR4/5/6 receptors. Genes Dev. 27, 1339–1344 (2013).
Deng, C. et al. Multi-functional norrin is a ligand for the LGR4 receptor. J. Cell Sci. 126, 2060–2068 (2013).
Tang, X. et al. GPR116, an adhesion G-protein-coupled receptor, promotes breast cancer metastasis via the Gαq-p63RhoGEF-Rho GTPase pathway. Cancer Res. 73, 6206–6218 (2013).
Clapham, D.E. Calcium signaling. Cell 131, 1047–1058 (2007).
Wu, X. et al. RANKL regulates Fas expression and Fas-mediated apoptosis in osteoclasts. J. Bone Miner. Res. 20, 107–116 (2005).
Krönke, G. et al. R-spondin 1 protects against inflammatory bone damage during murine arthritis by modulating the Wnt pathway. Arthritis Rheum. 62, 2303–2312 (2010).
Moon, J.B. et al. Akt induces osteoclast differentiation through regulating the GSK3β/NFATc1 signaling cascade. J. Immunol. 188, 163–169 (2012).
Takeshita, S. et al. Osteoclast-secreted CTHRC1 in the coupling of bone resorption to formation. J. Clin. Invest. 123, 3914–3924 (2013).
Mizuno, A. et al. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem. Biophys. Res. Commun. 247, 610–615 (1998).
Lacey, D.L. et al. Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am. J. Pathol. 157, 435–448 (2000).
Jimi, E. et al. Osteoclast differentiation factor acts as a multifunctional regulator in murine osteoclast differentiation and function. J. Immunol. 163, 434–442 (1999).
Boyce, B.F. Advances in the regulation of osteoclasts and osteoclast functions. J. Dent. Res. 92, 860–867 (2013).
Manolagas, S.C. & Parfitt, A.M. What old means to bone. Trends Endocrinol. Metab. 21, 369–374 (2010).
Pierce, B.G., Hourai, Y. & Weng, Z. Accelerating protein docking in ZDOCK using an advanced 3D convolution library. PLoS One 6, e24657 (2011).
Dai, W. et al. Improvement in low-homology template-based modeling by employing a model evaluation method with focus on topology. PLoS One 9, e89935 (2014).
Li, C. et al. Maslinic acid suppresses osteoclastogenesis and prevents ovariectomy-induced bone loss by regulating RANKL-mediated NF-κB and MAPK signaling pathways. J. Bone Miner. Res. 26, 644–656 (2011).
Chu, G.C. et al. RANK- and c-Met-mediated signal network promotes prostate cancer metastatic colonization. Endocr. Relat. Cancer 21, 311–326 (2014).
Wu, X. et al. Caffeic acid 3,4-dihydroxy-phenethyl ester suppresses receptor activator of NF-κB ligand–induced osteoclastogenesis and prevents ovariectomy-induced bone loss through inhibition of mitogen-activated protein kinase/activator protein 1 and Ca2+–nuclear factor of activated T-cells cytoplasmic 1 signaling pathways. J. Bone Miner. Res. 27, 1298–1308 (2012).
McMichael, B.K., Meyer, S.M. & Lee, B.S. c-Src-mediated phosphorylation of thyroid hormone receptor-interacting protein 6 (TRIP6) promotes osteoclast sealing zone formation. J. Biol. Chem. 285, 26641–26651 (2010).
Zhang, Z. et al. Ferroportin1 deficiency in mouse macrophages impairs iron homeostasis and inflammatory responses. Blood 118, 1912–1922 (2011).
Acknowledgements
This work is supported by grants from the National Basic Research Program of China (2012CB910402 to J.L.; 2012CB910400 to M.L.), the National Natural Science Foundation of China (81472048, 81272911 to J.L.; 81330049 to M.L.; 81522011 to J.W.), the Science and Technology Commission of Shanghai Municipality (15140903600 to J.L.), and the Innovation Program of Shanghai Municipal Education Commission (14ZZ051 to J.L.). The advanced ERC grant and an Era of Hope/DoD innovator award were given to JMP. We thank G. Ning (Ruijin Hospital, Shanghai JiaoTong University School of Medicine) for the Lgr4floxed mice, J. Penninger (Institute of Molecular Biotechnology of the Austrian Academy of Sciences) for the Rankfloxed mice, and Y. Zhang (Shanghai East Hospital, Tongji University School of Medicine) for the LysM-Cre mice.
Author information
Authors and Affiliations
Contributions
J.L. and Z. Yang generated the initial idea, proposed the hypothesis, and designed the study. Z.Yang conducted the key experiments. J.L. and M.L. supervised the study and performed the data analysis, interpreted results and wrote the manuscript. Y.M., Z. Yue, H.L., G.Q., J.H., C.L., and C.Z., performed the experiments. W.D. performed the docking and molecular modeling. L.X. and J.X. prepared and analyzed human samples. H.C., J.W., D.L., S.S., J.M.P., and G.N. provided the animals and analyzed the animal data. S.S. and J.M.P., analyzed data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 1–2 (PDF 4557 kb)
Rights and permissions
About this article
Cite this article
Luo, J., Yang, Z., Ma, Y. et al. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat Med 22, 539–546 (2016). https://doi.org/10.1038/nm.4076
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4076
This article is cited by
-
Recent advances of NFATc1 in rheumatoid arthritis-related bone destruction: mechanisms and potential therapeutic targets
Molecular Medicine (2024)
-
Transcriptional reprogramming during human osteoclast differentiation identifies regulators of osteoclast activity
Bone Research (2024)
-
Targeting strategies for bone diseases: signaling pathways and clinical studies
Signal Transduction and Targeted Therapy (2023)
-
SIRT2 regulates extracellular vesicle-mediated liver–bone communication
Nature Metabolism (2023)
-
Messages from the Mineral: How Bone Cells Communicate with Other Tissues
Calcified Tissue International (2023)