Abstract
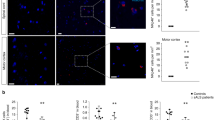
Astrocytes isolated from individuals with amyotrophic lateral sclerosis (ALS) are toxic to motor neurons (MNs) and play a non–cell autonomous role in disease pathogenesis. The mechanisms underlying the susceptibility of MNs to cell death remain unclear. Here we report that astrocytes derived from either mice bearing mutations in genes associated with ALS or human subjects with ALS reduce the expression of major histocompatibility complex class I (MHCI) molecules on MNs; reduced MHCI expression makes these MNs susceptible to astrocyte-induced cell death. Increasing MHCI expression on MNs increases survival and motor performance in a mouse model of ALS and protects MNs against astrocyte toxicity. Overexpression of a single MHCI molecule, HLA-F, protects human MNs from ALS astrocyte–mediated toxicity, whereas knockdown of its receptor, the killer cell immunoglobulin-like receptor KIR3DL2, on human astrocytes results in enhanced MN death. Thus, our data indicate that, in ALS, loss of MHCI expression on MNs renders them more vulnerable to astrocyte-mediated toxicity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hardiman, O., van den Berg, L.H. & Kiernan, M.C. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 7, 639–649 (2011).
Brown, R.H. Jr. Amyotrophic lateral sclerosis. Insights from genetics. Arch. Neurol. 54, 1246–1250 (1997).
Kwiatkowski, T.J. Jr. et al. Mutations in the FUS (TLS) gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323, 1205–1208 (2009).
Rosen, D.R. et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 (1993).
Sreedharan, J. et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319, 1668–1672 (2008).
Van Deerlin, V.M. et al. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurol. 7, 409–416 (2008).
Vance, C. et al. Mutations in FUS, an RNA-processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323, 1208–1211 (2009).
Bosco, D.A. et al. WT and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat. Neurosci. 13, 1396–1403 (2010).
Gruzman, A. et al. Common molecular signature in SOD1 for both sporadic and familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 104, 12524–12529 (2007).
Gurney, M.E. et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264, 1772–1775 (1994).
Ilieva, H., Polymenidou, M. & Cleveland, D.W. Non–cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J. Cell Biol. 187, 761–772 (2009).
Philips, T. & Rothstein, J.D. Glial cells in amyotrophic lateral sclerosis. Exp. Neurol. 262, 111–120 (2014).
Haidet-Phillips, A.M. et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat. Biotechnol. 29, 824–828 (2011).
Re, D.B. et al. Necroptosis drives motor neuron death in models of both sporadic and familial ALS. Neuron 81, 1001–1008 (2014).
Boulanger, L.M. Immune proteins in brain development and synaptic plasticity. Neuron 64, 93–109 (2009).
Tian, L., Ma, L., Kaarela, T. & Li, Z. Neuro-immune cross-talk in the central nervous system and its significance for neurological diseases. J. Neuroinflammation 9, 155 (2012).
Needleman, L.A., Liu, X.B., El-Sabeawy, F., Jones, E.G. & McAllister, A.K. MHC class I molecules are present both pre- and postsynaptically in the visual cortex during postnatal development and in adulthood. Proc. Natl. Acad. Sci. USA 107, 16999–17004 (2010).
Fourgeaud, L. et al. MHC class I modulates NMDA receptor function and AMPA receptor trafficking. Proc. Natl. Acad. Sci. USA 107, 22278–22283 (2010).
Goddard, C.A., Butts, D.A. & Shatz, C.J. Regulation of CNS synapses by neuronal MHC class I. Proc. Natl. Acad. Sci. USA 104, 6828–6833 (2007).
Huh, G.S. et al. Functional requirement for class I MHC in CNS development and plasticity. Science 290, 2155–2159 (2000).
Lee, H. et al. Synapse elimination and learning rules co-regulated by MHC class I H2-Db. Nature 509, 195–200 (2014).
McConnell, M.J., Huang, Y.H., Datwani, A. & Shatz, C.J. H2-Kb and H2-Db regulate cerebellar long-term depression and limit motor learning. Proc. Natl. Acad. Sci. USA 106, 6784–6789 (2009).
Freria, C.M., Zanon, R.G., Santos, L.M. & Oliveira, A.L. Major histocompatibility complex class I expression and glial reaction influence spinal motoneuron synaptic plasticity during the course of experimental autoimmune encephalomyelitis. J. Comp. Neurol. 518, 990–1007 (2010).
Kim, T. et al. Human LILRB2 is a β-amyloid receptor and its murine homolog PirB regulates synaptic plasticity in an Alzheimer's model. Science 341, 1399–1404 (2013).
Nardo, G. et al. Transcriptomic indices of fast and slow disease progression in two mouse models of amyotrophic lateral sclerosis. Brain 136, 3305–3332 (2013).
Staats, K.A. et al. β-2 microglobulin is important for disease progression in a murine model for amyotrophic lateral sclerosis. Front. Cell. Neurosci. 7, 249 (2013).
Lindå, H., Hammarberg, H., Piehl, F., Khademi, M. & Olsson, T. Expression of MHC class I heavy chain and β2-microglobulin in rat brainstem motoneurons and nigral dopaminergic neurons. J. Neuroimmunol. 101, 76–86 (1999).
Frakes, A.E. et al. Microglia induce motor neuron death via the classical NF-κB pathway in amyotrophic lateral sclerosis. Neuron 81, 1009–1023 (2014).
Miranda, C.J. et al. Aging brain microenvironment decreases hippocampal neurogenesis through Wnt-mediated survivin signaling. Aging Cell 11, 542–552 (2012).
Clarke, L.E. & Barres, B.A. Emerging roles of astrocytes in neural circuit development. Nat. Rev. Neurosci. 14, 311–321 (2013).
Liu, J. et al. The expression pattern of classical MHC class I molecules in the development of mouse central nervous system. Neurochem. Res. 38, 290–299 (2013).
Israelson, A. et al. Macrophage migration inhibitory factor as a chaperone inhibiting accumulation of misfolded SOD1. Neuron 86, 218–232 (2015).
Marchetto, M.C. et al. Non–cell autonomous effect of human SOD1G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell 3, 649–657 (2008).
Nagai, M. et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat. Neurosci. 10, 615–622 (2007).
Nishitoh, H. et al. ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting Derlin-1. Genes Dev. 22, 1451–1464 (2008).
Dodge, J.C. et al. Delivery of AAV-IGF1 to the CNS extends survival in ALS mice through modification of aberrant glial cell activity. Mol. Ther. 16, 1056–1064 (2008).
Chakrabarty, P. et al. Capsid serotype and timing of injection determine AAV transduction in the neonatal mouse brain. PLoS One 8, e67680 (2013).
Robbins, K.L., Glascock, J.J., Osman, E.Y., Miller, M.R. & Lorson, C.L. Defining the therapeutic window in a severe animal model of spinal muscular atrophy. Hum. Mol. Genet. 23, 4559–4568 (2014).
Tay, C.H., Szomolanyi-Tsuda, E. & Welsh, R.M. Control of infections by NK cells. Curr. Top. Microbiol. Immunol. 230, 193–220 (1998).
Lanier, L.L. NK cell recognition. Annu. Rev. Immunol. 23, 225–274 (2005).
Long, E.O. Regulation of immune responses through inhibitory receptors. Annu. Rev. Immunol. 17, 875–904 (1999).
Chiu, I.M. et al. T lymphocytes potentiate endogenous neuroprotective inflammation in a mouse model of ALS. Proc. Natl. Acad. Sci. USA 105, 17913–17918 (2008).
Goodridge, J.P., Burian, A., Lee, N. & Geraghty, D.E. HLA-F and MHC class I open conformers are ligands for NK cell Ig-like receptors. J. Immunol. 191, 3553–3562 (2013).
Meyer, K. et al. Direct conversion of patient fibroblasts demonstrates non–cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. Proc. Natl. Acad. Sci. USA 111, 829–832 (2014).
Oliveira, A.L. et al. A role for MHC class I molecules in synaptic plasticity and regeneration of neurons after axotomy. Proc. Natl. Acad. Sci. USA 101, 17843–17848 (2004).
Thams, S. et al. Classical major histocompatibility complex class I molecules in motoneurons: new actors at the neuromuscular junction. J. Neurosci. 29, 13503–13515 (2009).
Rivera-Quiñones, C. et al. Absence of neurological deficits following extensive demyelination in a class I–deficient murine model of multiple sclerosis. Nat. Med. 4, 187–193 (1998).
Hansen, T.H. & Bouvier, M. MHC class I antigen presentation: learning from viral evasion strategies. Nat. Rev. Immunol. 9, 503–513 (2009).
Saxena, S., Cabuy, E. & Caroni, P. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat. Neurosci. 12, 627–636 (2009).
Filézac de L'Etang, A. et al. Marinesco-Sjögren syndrome protein SIL1 regulates motor neuron subtype–selective ER stress in ALS. Nat. Neurosci. 18, 227–238 (2015).
Lautenschlaeger, J., Prell, T. & Grosskreutz, J. Endoplasmic reticulum stress and the ER mitochondrial calcium cycle in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 13, 166–177 (2012).
Kikuchi, H. et al. Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase 1 in an ALS model. Proc. Natl. Acad. Sci. USA 103, 6025–6030 (2006).
Díaz-Amarilla, P. et al. Phenotypically aberrant astrocytes that promote motoneuron damage in a model of inherited amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 108, 18126–18131 (2011).
Moscoso, J., Serrano-Vela, J.I., Pacheco, R. & Arnaiz-Villena, A. HLA-G, HLA-E and HLA-F: allelism, function and evolution. Transpl. Immunol. 17, 61–64 (2006).
Bevan, A.K. et al. Systemic gene delivery in large species for targeting spinal cord, brain and peripheral tissues for pediatric disorders. Mol. Ther. 19, 1971–1980 (2011).
Foust, K.D. et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 27, 59–65 (2009).
Foust, K.D. et al. Therapeutic AAV9-mediated suppression of mutant SOD1 slows disease progression and extends survival in models of inherited ALS. Mol. Ther. 21, 2148–2159 (2013).
Miyazaki, Y. et al. Viral delivery of miR-196a ameliorates the SBMA phenotype via the silencing of CELF2. Nat. Med. 18, 1136–1141 (2012).
Meyer, K. et al. Improving single-injection CSF delivery of AAV9-mediated gene therapy for SMA: a dose-response study in mice and nonhuman primates. Mol. Ther. 23, 477–487 (2015).
Leitner, M., Menzies, S. & Lutz, C. Working with ALS Mice: Guidelines for Preclinical Testing and Colony Management (The Jackson Laboratory, Bar Harbor, Maine, USA, 2009).
Noble, M. & Mayer-Proschel, M. Culture of Astrocytes, Oligodendrocytes and O-2A Progenitor Cells (MIT press, Cambridge, 1998).
Ray, J. & Gage, F.H. Differential properties of adult rat and mouse brain–derived neural stem or progenitor cells. Mol. Cell. Neurosci. 31, 560–573 (2006).
Hester, M.E. et al. Two-factor reprogramming of human neural stem cells into pluripotency. PLoS One 4, e7044 (2009).
Kim, J.B. et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature 454, 646–650 (2008).
Kaech, S. & Banker, G. Culturing hippocampal neurons. Nat. Protoc. 1, 2406–2415 (2006).
Hester, M.E. et al. Rapid and efficient generation of functional motor neurons from human pluripotent stem cells by using gene-delivered transcription factor codes. Mol. Ther. 19, 1905–1912 (2011).
Ludolph, A.C. et al. Guidelines for preclinical animal research in ALS/MND: a consensus meeting. Amyotroph. Lateral Scler. 11, 38–45 (2010).
Thompson, A., van der Slik, A.R., Koning, F. & van Bergen, J. An improved RT-PCR method for the detection of killer cell immunoglobulin-like receptor (KIR) transcripts. Immunogenetics 58, 865–872 (2006).
Syken, J. & Shatz, C.J. Expression of T cell receptor beta locus in central nervous system neurons. Proc. Natl. Acad. Sci. USA 100, 13048–13053 (2003).
Ishogami, S. et al. Clinical-pathological implication of human leukocyte antigen–F–positive gastric adenocarcinoma. J. Surg. Res. 184, 802–806 (2013).
Zhang, J.G. et al. Lesion HLA-F expression is irrelevant to prognosis for patients with gastric cancer. Hum. Immunol. 74, 828–832 (2013).
Acknowledgements
We thank C. Shatz (Stanford University) for critical discussions; S. Eckardt for expert editorial assistance; the National Disease Research Institute (NDRI), A. Burghes (Ohio State University), J.R. Mendell (Nationwide Children's Hospital), J. Glass and M. Gearing (Emory University; supported by US National Institutes of Health (NIH)-NINDS grant no. P30NS055077) for providing human spinal cord specimens; F. Gage (Salk Institute) for providing human post-mortem neural progenitor cells (NPCs) that were used to generate non-ALS astrocytes; M. Hester for guidance with iPSC generation; T. Jessell (Columbia University) for HBG3 cells; and K. Campbell for technical assistance. This work was supported by NIH grants R01-NS644912 (B.K.K.) and RC2-NS69476 (B.K.K.), funding from the Robert Packard Center for ALS Research (B.K.K.), the Project A.L.S. (B.K.K.) and the Helping Link Foundation (B.K.K.). The authors that were a part of this work also received research fellowships from the Swiss National Science Foundation (K.M.), the Marie Curie Foundation (L.F.), the NINDS Training in Neuromuscular Disease (A.E.F.), the Ohio State University Presidential Fellowship (S.W.S.) and the Ohio State University and Nationwide Children's Hospital Muscle Group Fellowship (S.W.S.).
Author information
Authors and Affiliations
Contributions
All authors contributed to the design of the experiments. Isolation of mouse astrocytes, microglia and NPCs was performed by S.W.S., C.J.M., L.B., A.E.F. and S.L.; human astrocyte cultures were done by S.W.S., C.J.M., K.M. and L.F.; neuronal cell differentiation and coculture experiments were performed by S.W.S., C.J.M., K.M., A.E.F. and S.L.; in situ hybridization of H2d transcripts was performed by M.J.M.; RT-PCR and immunocytochemistry analyses were done by S.W.S., C.J.M., L.B. and A.K.B.; lentivirus production was done by S.W.S., C.J.M., L.B., K.M., A.E.F., L.F. and S.L.; and data was analyzed by S.W.S., C.J.M., L.B., K.M., A.E.F., L.F., S.L., A.K.B., K.D.F., M.J.M., C.M.W. and B.K.K. The manuscript and figures were prepared by S.W.S., C.J.M. and B.K.K., with input from all coauthors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 and Supplementary Tables 1–4 (PDF 2496 kb)
Rights and permissions
About this article
Cite this article
Song, S., Miranda, C., Braun, L. et al. Major histocompatibility complex class I molecules protect motor neurons from astrocyte-induced toxicity in amyotrophic lateral sclerosis. Nat Med 22, 397–403 (2016). https://doi.org/10.1038/nm.4052
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.4052
This article is cited by
-
Janus kinase inhibitors are potential therapeutics for amyotrophic lateral sclerosis
Translational Neurodegeneration (2023)
-
Evolution and molecular interactions of major histocompatibility complex (MHC)-G, -E and -F genes
Cellular and Molecular Life Sciences (2022)
-
Analysis of whole exome sequencing in severe mental illness hints at selection of brain development and immune related genes
Scientific Reports (2021)
-
Correlation of alteration of HLA-F expression and clinical characterization in 593 brain glioma samples
Journal of Neuroinflammation (2019)
-
Friends Turn Foe—Astrocytes Contribute to Neuronal Damage in NeuroAIDS
Journal of Molecular Neuroscience (2019)