Abstract
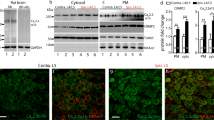
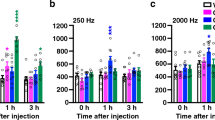
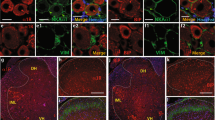
Mechanical allodynia, induced by normally innocuous low-threshold mechanical stimulation, represents a cardinal feature of neuropathic pain. Blockade or ablation of high-threshold, small-diameter unmyelinated group C nerve fibers (C-fibers) has limited effects on mechanical allodynia1,2,3,4. Although large, myelinated group A fibers, in particular Aβ-fibers, have previously been implicated in mechanical allodynia5,6,7, an A-fiber–selective pharmacological blocker is still lacking. Here we report a new method for targeted silencing of A-fibers in neuropathic pain. We found that Toll-like receptor 5 (TLR5) is co-expressed with neurofilament-200 in large-diameter A-fiber neurons in the dorsal root ganglion (DRG). Activation of TLR5 with its ligand flagellin results in neuronal entry of the membrane-impermeable lidocaine derivative QX-314, leading to TLR5-dependent blockade of sodium currents, predominantly in A-fiber neurons of mouse DRGs. Intraplantar co-application of flagellin and QX-314 (flagellin/QX-314) dose-dependently suppresses mechanical allodynia after chemotherapy, nerve injury, and diabetic neuropathy, but this blockade is abrogated in Tlr5-deficient mice. In vivo electrophysiology demonstrated that co-application of flagellin/QX-314 selectively suppressed Aβ-fiber conduction in naive and chemotherapy-treated mice. TLR5-mediated Aβ-fiber blockade, but not capsaicin-mediated C-fiber blockade, also reduced chemotherapy-induced ongoing pain without impairing motor function. Finally, flagellin/QX-314 co-application suppressed sodium currents in large-diameter human DRG neurons. Thus, our findings provide a new tool for targeted silencing of Aβ-fibers and neuropathic pain treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ossipov, M.H., Bian, D., Malan, T.P. Jr., Lai, J. & Porreca, F. Lack of involvement of capsaicin-sensitive primary afferents in nerve-ligation injury induced tactile allodynia in rats. Pain 79, 127–133 (1999).
Minett, M.S. et al. Pain without nociceptors? Nav1.7-independent pain mechanisms. Cell Rep. 6, 301–312 (2014).
Liu, C.N. et al. Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain 85, 503–521 (2000).
Brenneis, C. et al. Phenotyping the function of TRPV1-expressing sensory neurons by targeted axonal silencing. J. Neurosci. 33, 315–326 (2013).
Koltzenburg, M., Lundberg, L.E. & Torebjork, H.E. Dynamic and static components of mechanical hyperalgesia in human hairy skin. Pain 51, 207–219 (1992).
Ossipov, M.H. et al. Selective mediation of nerve injury-induced tactile hypersensitivity by neuropeptide Y. J. Neurosci. 22, 9858–9867 (2002).
Campbell, J.N., Raja, S.N., Meyer, R.A. & Mackinnon, S.E. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain 32, 89–94 (1988).
Hayashi, F. et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410, 1099–1103 (2001).
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Liu, T., Gao, Y.J. & Ji, R.R. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci. Bull. 28, 131–144 (2012).
Liu, T. et al. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J. Clin. Invest. 122, 2195–2207 (2012).
Lawson, S.N. & Waddell, P.J. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J. Physiol. (Lond.) 435, 41–63 (1991).
McCoy, E.S. et al. Peptidergic CGRPα primary sensory neurons encode heat and itch and tonically suppress sensitivity to cold. Neuron 78, 138–151 (2013).
Li, L. et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 147, 1615–1627 (2011).
Todd, A.J. Neuronal circuitry for pain processing in the dorsal horn. Nat. Rev. Neurosci. 11, 823–836 (2010).
Binshtok, A.M., Bean, B.P. & Woolf, C.J. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature 449, 607–610 (2007).
Roberson, D.P. et al. Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nat. Neurosci. 16, 910–918 (2013).
Park, C.K. et al. Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron 82, 47–54 (2014).
Smith, K.D. et al. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 4, 1247–1253 (2003).
Liu, X.J. et al. Nociceptive neurons regulate innate and adaptive immunity and neuropathic pain through MyD88 adapter. Cell Res. 24, 1374–1377 (2014).
Cavaletti, G. & Marmiroli, P. Chemotherapy-induced peripheral neurotoxicity. Nat. Rev. Neurol. 6, 657–666 (2010).
Binshtok, A.M. et al. Nociceptors are interleukin-1β sensors. J. Neurosci. 28, 14062–14073 (2008).
King, T. et al. Contribution of afferent pathways to nerve injury-induced spontaneous pain and evoked hypersensitivity. Pain 152, 1997–2005 (2011).
Xu, Z.Z. et al. Neuroprotectin/protectin D1 protects neuropathic pain in mice after nerve trauma. Ann. Neurol. 74, 490–495 (2013).
Khan, G.M., Chen, S.R. & Pan, H.L. Role of primary afferent nerves in allodynia caused by diabetic neuropathy in rats. Neuroscience 114, 291–299 (2002).
Bennett, G.J. & Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33, 87–107 (1988).
Melzack, R. & Wall, P.D. Pain mechanisms: a new theory. Science 150, 971–979 (1965).
Usoskin, D. et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 18, 145–153 (2015).
Devor, M. Neuropathic pain and injured nerve: peripheral mechanisms. Br. Med. Bull. 47, 619–630 (1991).
Ma, C. et al. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J. Neurophysiol. 89, 1588–1602 (2003).
Haroutounian, S. et al. Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy. Pain 155, 1272–1279 (2014).
Kim, Y.H. et al. TRPV1 in GABAergic interneurons mediates neuropathic mechanical allodynia and disinhibition of the nociceptive circuitry in the spinal cord. Neuron 74, 640–647 (2012).
Seal, R.P. et al. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature 462, 651–655 (2009).
Boada, M.D. et al. Fast-conducting mechanoreceptors contribute to withdrawal behavior in normal and nerve injured rats. Pain 155, 2646–2655 (2014).
Smith, A.K., O'Hara, C.L. & Stucky, C.L. Mechanical sensitization of cutaneous sensory fibers in the spared nerve injury mouse model. Mol. Pain 9, 61 (2013).
Lu, Y. et al. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J. Clin. Invest. 123, 4050–4062 (2013).
Duan, B. et al. Identification of spinal circuits transmitting and gating mechanical pain. Cell 159, 1417–1432 (2014).
Vijay-Kumar, M. et al. Flagellin treatment protects against chemicals, bacteria, viruses, and radiation. J. Immunol. 180, 8280–8285 (2008).
Sfondrini, L. et al. Antitumor activity of the TLR-5 ligand flagellin in mouse models of cancer. J. Immunol. 176, 6624–6630 (2006).
Alessandri-Haber, N., Dina, O.A., Joseph, E.K., Reichling, D.B. & Levine, J.D. Interaction of transient receptor potential vanilloid 4, integrin, and SRC tyrosine kinase in mechanical hyperalgesia. J. Neurosci. 28, 1046–1057 (2008).
Berta, T. et al. Extracellular caspase-6 drives murine inflammatory pain via microglial TNF-α secretion. J. Clin. Invest. 124, 1173–1186 (2014).
Pagadala, P. et al. Loss of NR1 subunit of NMDARs in primary sensory neurons leads to hyperexcitability and pain hypersensitivity: involvement of Ca2+-activated small conductance potassium channels. J. Neurosci. 33, 13425–13430 (2013).
Liu, T., Xu, Z.Z., Park, C.K., Berta, T. & Ji, R.R. Toll-like receptor 7 mediates pruritus. Nat. Neurosci. 13, 1460–1462 (2010).
Xie, R.G. et al. Blockade of persistent sodium currents contributes to the riluzole-induced inhibition of spontaneous activity and oscillations in injured DRG neurons. PLoS ONE 6, e18681 (2011).
Lee, J.H. et al. A monoclonal antibody that targets a NaV1.7 channel voltage sensor for pain and itch relief. Cell 157, 1393–1404 (2014).
Baumann, T.K., Chaudhary, P. & Martenson, M.E. Background potassium channel block and TRPV1 activation contribute to proton depolarization of sensory neurons from humans with neuropathic pain. Eur. J. Neurosci. 19, 1343–1351 (2004).
Davidson, S. et al. Human sensory neurons: Membrane properties and sensitization by inflammatory mediators. Pain 155, 1861–1870 (2014).
Pinto, V., Derkach, V.A. & Safronov, B.V. Role of TTX-sensitive and TTX-resistant sodium channels in Adelta- and C-fiber conduction and synaptic transmission. J. Neurophysiol. 99, 617–628 (2008).
Tsuchimochi, H., McCord, J.L., Leal, A.K. & Kaufman, M.P. Dorsal root tetrodotoxin-resistant sodium channels do not contribute to the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. Am. J. Physiol. Heart Circ. Physiol. 300, H652–H663 (2011).
Dixon, W.J. Efficient analysis of experimental observations. Annu. Rev. Pharmacol. Toxicol. 20, 441–462 (1980).
Hargreaves, K., Dubner, R., Brown, F., Flores, C. & Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32, 77–88 (1988).
King, T. et al. Unmasking the tonic-aversive state in neuropathic pain. Nat. Neurosci. 12, 1364–1366 (2009).
Park, C.K. et al. Resolving TRPV1- and TNF-α-mediated spinal cord synaptic plasticity and inflammatory pain with neuroprotectin D1. J. Neurosci. 31, 15072–15085 (2011).
Acknowledgements
This study is supported by US National Institutes of Health (NIH) R01 grants NS67686 (R.-R.J.), NS87988 (R.-R.J.), NS89479 (R.-R.J.), DE17794 (R.-R.J.), and DE22743 (R.-R.J.); NIH R21 grants NS82985 (Z.-Z.X.) and NS91779 (Z.-Z.X.); NIH R01 grant DE19440 (F.W.); a Korea government grant 2012R1A3A2048834 (S.B.O.); and a Korea National Research Foundation grant 2013R1A6A3A04065858 (Y.H.K.).
Author information
Authors and Affiliations
Contributions
Z.-Z.X. developed the behavioral part of the project and designed and performed immunohistochemical and behavioral experiments. Y.H.K. recorded action potentials in mouse DRG neurons and compound potentials in intact mice, and recorded sodium currents in human DRG neurons. S.B. initially tested the idea of flagellin/QX-314 blockade of sodium currents in mouse DRG neurons and examined dye (phallodin-rhodamine) uptake in DRGs. Y.Z. performed in situ hybridization under the guidance of F.W. T.B. performed the PCR experiments. R.-R.J. conceived and supervised the project. S.B.O. and F.W. discussed the project; and R.-R.J., Z.-Z.X., S.B.O., and F.W. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Table 1 and Supplementary Figures 1–12 (PDF 5037 kb)
Source data
Rights and permissions
About this article
Cite this article
Xu, ZZ., Kim, Y., Bang, S. et al. Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat Med 21, 1326–1331 (2015). https://doi.org/10.1038/nm.3978
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3978
This article is cited by
-
Endophilin A2 controls touch and mechanical allodynia via kinesin-mediated Piezo2 trafficking
Military Medical Research (2024)
-
An ACC–VTA–ACC positive-feedback loop mediates the persistence of neuropathic pain and emotional consequences
Nature Neuroscience (2024)
-
Hallmarks of peripheral nerve function in bone regeneration
Bone Research (2023)
-
Synchronized activity of sensory neurons initiates cortical synchrony in a model of neuropathic pain
Nature Communications (2023)
-
Hypersensitivity of myelinated A-fibers via toll-like receptor 5 promotes mechanical allodynia in tenascin-X-deficient mice associated with Ehlers–Danlos syndrome
Scientific Reports (2023)