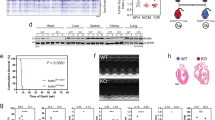
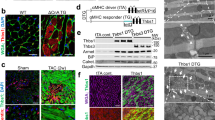
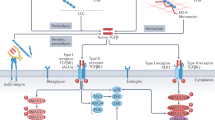
Abstract
Tumor necrosis factor-α (TNF-α), one of the major stress-induced proinflammatory cytokines, is upregulated in the heart after tissue injury1,2, and its sustained expression can contribute to the development of heart failure1,3,4. Whether TNF-α also exerts cytoprotective effects in heart failure is not known. Here we provide evidence for a cardioprotective function of TNF-α in a genetic heart failure model, desmin-deficient mice. The cardioprotective effects of TNF-α are a consequence of nuclear factor-κB (NF-κB)-mediated ectopic expression in cardiomyocytes of keratin 8 (K8) and keratin 18 (K18), two epithelial-specific intermediate filament proteins5,6. In cardiomyocytes, K8 and K18 (K8/K18) formed an alternative cytoskeletal network that localized mainly at intercalated discs (IDs) and conferred cardioprotection by maintaining normal ID structure and mitochondrial integrity and function. Ectopic induction of K8/K18 expression in cardiomyocytes also occurred in other genetic and experimental models of heart failure. Loss of the K8/K18 network resulted in a maladaptive cardiac phenotype following transverse aortic constriction. In human failing myocardium, where TNF-α expression is upregulated2, K8/K18 were also ectopically expressed and localized primarily at IDs, which did not contain detectable amounts of desmin. Thus, TNF-α– and NF-κB-mediated formation of an alternative, stress-induced intermediate filament cytoskeleton has cardioprotective function in mice and potentially in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Mann, D.L. Stress-activated cytokines and the heart: from adaptation to maladaptation. Annu. Rev. Physiol. 65, 81–101 (2003).
Levine, B., Kalman, J., Mayer, L., Fillit, H.M. & Packer, M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N. Engl. J. Med. 323, 236–241 (1990).
Sivasubramanian, N. et al. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation 104, 826–831 (2001).
Pagani, F.D. et al. Left ventricular systolic and diastolic dysfunction after infusion of tumor necrosis factor-α in conscious dogs. J. Clin. Invest. 90, 389–398 (1992).
Omary, M.B., Ku, N.O., Strnad, P. & Hanada, S. Toward unraveling the complexity of simple epithelial keratins in human disease. J. Clin. Invest. 119, 1794–1805 (2009).
Pan, X., Hobbs, R.P. & Coulombe, P.A. The expanding significance of keratin intermediate filaments in normal and diseased epithelia. Curr. Opin. Cell Biol. 25, 47–56 (2013).
Durán, W.N. The double-edge sword of TNF-α in ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 295, H2221–H2222 (2008).
Kapadia, S.R. et al. Elevated circulating levels of serum tumor necrosis factor-α in patients with hemodynamically significant pressure and volume overload. J. Am. Coll. Cardiol. 36, 208–212 (2000).
Burchfield, J.S. et al. The cytoprotective effects of tumor necrosis factor are conveyed through tumor necrosis factor receptor-associated factor 2 in the heart. Circ Heart Fail 3, 157–164 (2010).
Kurrelmeyer, K.M. et al. Endogenous tumor necrosis factor protects the adult cardiac myocyte against ischemic-induced apoptosis in a murine model of acute myocardial infarction. Proc. Natl. Acad. Sci. USA 97, 5456–5461 (2000).
Feldman, A.M., Kadokami, T., Higuichi, Y., Ramani, R. & McTiernan, C.F. The role of anticytokine therapy in heart failure: recent lessons from preclinical and clinical trials? Med. Clin. North Am. 87, 419–440 (2003).
Haudek, S.B., Taffet, G.E., Schneider, M.D. & Mann, D.L. TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways. J. Clin. Invest. 117, 2692–2701 (2007).
Panagopoulou, P. et al. Desmin mediates TNF-α-induced aggregate formation and intercalated disk reorganization in heart failure. J. Cell Biol. 181, 761–775 (2008).
Capetanaki, Y., Papathanasiou, S., Diokmetzidou, A., Vatsellas, G. & Tsikitis, M. Desmin related disease: a matter of cell survival failure. Curr. Opin. Cell Biol. 32, 113–120 (2015).
Goldfarb, L.G. & Dalakas, M.C. Tragedy in a heartbeat: malfunctioning desmin causes skeletal and cardiac muscle disease. J. Clin. Invest. 119, 1806–1813 (2009).
Milner, D.J., Weitzer, G., Tran, D., Bradley, A. & Capetanaki, Y. Disruption of muscle architecture and myocardial degeneration in mice lacking desmin. J. Cell Biol. 134, 1255–1270 (1996).
Psarras, S. et al. Regulation of adverse remodelling by osteopontin in a genetic heart failure model. Eur. Heart J. 33, 1954–1963 (2012).
Mavroidis, M. et al. Complement system modulation as a target for treatment of arrhythmogenic cardiomyopathy. Basic Res. Cardiol. 110, 27 (2015).
Milner, D.J., Mavroidis, M., Weisleder, N. & Capetanaki, Y. Desmin cytoskeleton linked to muscle mitochondrial distribution and respiratory function. J. Cell Biol. 150, 1283–1298 (2000).
Moll, R., Franke, W.W., Schiller, D.L., Geiger, B. & Krepler, R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31, 11–24 (1982).
Kuruc, N. & Franke, W.W. Transient coexpression of desmin and cytokeratins 8 and 18 in developing myocardial cells of some vertebrate species. Differentiation 38, 177–193 (1988).
Gown, A.M. & Vogel, A.M. Monoclonal antibodies to human intermediate filament proteins. II. Distribution of filament proteins in normal human tissues. Am. J. Pathol. 114, 309–321 (1984).
Ursitti, J.A. et al. Cloning and characterization of cytokeratins 8 and 19 in adult rat striated muscle. Interaction with the dystrophin glycoprotein complex. J. Biol. Chem. 279, 41830–41838 (2004).
Maier, H.J. et al. Cardiomyocyte-specific IκB kinase (IKK)/NF-κB activation induces reversible inflammatory cardiomyopathy and heart failure. Proc. Natl. Acad. Sci. USA 109, 11794–11799 (2012).
Brown, M. et al. Cardiac-specific blockade of NF-κB in cardiac pathophysiology: differences between acute and chronic stimuli in vivo. Am. J. Physiol. Heart Circ. Physiol. 289, H466–H476 (2005).
Franke, W.W., Borrmann, C.M., Grund, C. & Pieperhoff, S. The area composita of adhering junctions connecting heart muscle cells of vertebrates. I. Molecular definition in intercalated disks of cardiomyocytes by immunoelectron microscopy of desmosomal proteins. Eur. J. Cell Biol. 85, 69–82 (2006).
Kim, S. & Coulombe, P.A. Intermediate filament scaffolds fulfill mechanical, organizational, and signaling functions in the cytoplasm. Genes Dev. 21, 1581–1597 (2007).
Magin, T.M. et al. Lessons from keratin 18 knockout mice: formation of novel keratin filaments, secondary loss of keratin 7 and accumulation of liver-specific keratin 8-positive aggregates. J. Cell Biol. 140, 1441–1451 (1998).
Tao, G.Z. et al. Keratins modulate the shape and function of hepatocyte mitochondria: a mechanism for protection from apoptosis. J. Cell Sci. 122, 3851–3855 (2009).
Loranger, A., Gilbert, S., Brouard, J.S., Magin, T.M. & Marceau, N. Keratin 8 modulation of desmoplakin deposition at desmosomes in hepatocytes. Exp. Cell Res. 312, 4108–4119 (2006).
Kröger, C. et al. Keratins control intercellular adhesion involving PKC-α-mediated desmoplakin phosphorylation. J. Cell Biol. 201, 681–692 (2013).
Green, K.J. & Gaudry, C.A. Are desmosomes more than tethers for intermediate filaments? Nat. Rev. Mol. Cell Biol. 1, 208–216 (2000).
Capetanaki, Y. Desmin cytoskeleton: a potential regulator of muscle mitochondrial behavior and function. Trends Cardiovasc. Med. 12, 339–348 (2002).
Toivola, D.M., Tao, G.Z., Habtezion, A., Liao, J. & Omary, M.B. Cellular integrity plus: organelle-related and protein-targeting functions of intermediate filaments. Trends Cell Biol. 15, 608–617 (2005).
Fountoulakis, M. et al. Alterations in the heart mitochondrial proteome in a desmin null heart failure model. J. Mol. Cell. Cardiol. 38, 461–474 (2005).
Duan, S. et al. The Pirh2-keratin 8/18 interaction modulates the cellular distribution of mitochondria and UV-induced apoptosis. Cell Death Differ. 16, 826–837 (2009).
Stone, M.R. et al. Absence of keratin 19 in mice causes skeletal myopathy with mitochondrial and sarcolemmal reorganization. J. Cell Sci. 120, 3999–4008 (2007).
Molkentin, J.D. et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93, 215–228 (1998).
Arber, S. et al. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell 88, 393–403 (1997).
Kaplan, S.R. et al. Structural and molecular pathology of the heart in Carvajal syndrome. Cardiovasc. Pathol. 13, 26–32 (2004).
Li, X. et al. Cardiac-specific overexpression of tumor necrosis factor-α causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation 102, 1690–1696 (2000).
Huss, J.M. et al. The nuclear receptor ERRα is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab. 6, 25–37 (2007).
Rickelt, S., Moll, I. & Franke, W.W. Intercellular adhering junctions with an asymmetric molecular composition: desmosomes connecting Merkel cells and keratinocytes. Cell Tissue Res. 346, 65–77 (2011).
García-Nogales, P., Almeida, A., Fernandez, E., Medina, J.M. & Bolanos, J.P. Induction of glucose-6-phosphate dehydrogenase by lipopolysaccharide contributes to preventing nitric oxide-mediated glutathione depletion in cultured rat astrocytes. J. Neurochem. 72, 1750–1758 (1999).
Edgar, R., Domrachev, M. & Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 (2002).
Nakamichi, I. et al. Keratin 8 overexpression promotes mouse Mallory body formation. J. Cell Biol. 171, 931–937 (2005).
Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45 (2001).
van den Boogaard, M., Wong, L.Y., Christoffels, V.M. & Barnett, P. Acquisition of high quality DNA for massive parallel sequencing by in vivo chromatin immunoprecipitation. Methods Mol. Biol. 977, 53–64 (2013).
Kent, W.J. et al. The human genome browser at UCSC. Genome Res. 12, 996–1006 (2002).
Myers, R.M. et al. A user's guide to the encyclopedia of DNA elements (ENCODE). PLoS Biol. 9, e1001046 (2011).
Rosenbloom, K.R. et al. ENCODE data in the UCSC Genome Browser: year 5 update. Nucleic Acids Res. 41, D56–D63 (2013).
Sabo, P.J. et al. Genome-scale mapping of DNase I sensitivity in vivo using tiling DNA microarrays. Nat. Methods 3, 511–518 (2006).
Sabo, P.J. et al. Discovery of functional noncoding elements by digital analysis of chromatin structure. Proc. Natl. Acad. Sci. USA 101, 16837–16842 (2004).
Antonaki, A. et al. Genomic analysis reveals a novel nuclear factor-κB (NF-κB)-binding site in Alu-repetitive elements. J. Biol. Chem. 286, 38768–38782 (2011).
Kaltezioti, V. et al. Prox1 regulates the notch1-mediated inhibition of neurogenesis. PLoS Biol. 8, e1000565 (2010).
Acknowledgements
We thank W. Franke and Y. Dörflinger (Helmholtz Group for Cell Biology, German Cancer Research Center, Heidelberg) for extensive technical support with EM and immunogold labeling, for providing EM-related materials, antibodies and human tissue samples, and for very valuable comments. We especially thank L. Scorrano (University of Padova) for his help and support on the study of mitochondrial function and for providing the consumables for mitochondrial function experiments for these experiments. We thank L. Staloch, C. Weinheimer and A. Kovachs for the TAC surgeries and echocarardiographic measurements of the TACed mice. We also thank W.K. Jones (Department of Pharmacology and Cell Biophysics, University of Cincinnati) for kindly providing the IκBα-3M mice, M. Gerstenlauer for technical assistance with IKKMyHC mice experiments and J. D. Molkentin (Howard Hughes Medical Institute and Cincinnati Children's Hospital Medical Center) for providing samples of MHC-Cn and Csrp3−/− mice and mice subjected to TAC or MI. We also thank P. Politis and N. Athanassiadis (Biomedical Research Foundation, Academy of Athens) for providing materials related to the luciferase reporter assays and human samples, respectively. Our sincere thanks to I. Kostavasili for her long-term technical assistance throughout this project. This work was supported by the Greek Secretariat of Research and Development grants (PENED 01ED371, PEP-ATT-39, ESPA SYNERGASIA 09SYN-21-965) and grant of “Excellence II” 5342 to Y.C., a US National Institutes of Health R01 HL 111094 grant to D.L.M. and a fellowship (Heracleitus II) by the European Union and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework to S.P.
Author information
Authors and Affiliations
Contributions
Y.C. and S.P. conceived the project, designed research and analyzed all the data. S.P. performed the research. S.R. helped with the EM experiments. M.E.S. helped with the mitochondrial functional experiments. T.G.S. helped with the experiments on several models of heart failure. C.H.D. and A.V. helped with echocardiography. H.J.M. contributed with analyses of the IKKMyHC mice. L.K. provided the human samples. D.L.M. provided the original TnfMyh6 mice, helped with TAC experiments and data interpretation. S.P. and Y.C. wrote the paper, and Y.C. supervised all aspects of the work. All co-authors approved the submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 & Supplementary Tables 1–3 (PDF 27007 kb)
Dataset 1
GO analysis of the mostly altered genes in TnfMyh6Des−/− myocardium. (XLS 71 kb)
Rights and permissions
About this article
Cite this article
Papathanasiou, S., Rickelt, S., Soriano, M. et al. Tumor necrosis factor-α confers cardioprotection through ectopic expression of keratins K8 and K18. Nat Med 21, 1076–1084 (2015). https://doi.org/10.1038/nm.3925
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3925
This article is cited by
-
Altered acylcarnitine metabolism and inflexible mitochondrial fuel utilization characterize the loss of neonatal myocardial regeneration capacity
Experimental & Molecular Medicine (2023)
-
The potential of remdesivir to affect function, metabolism and proliferation of cardiac and kidney cells in vitro
Archives of Toxicology (2022)
-
Deficiency of myostatin protects skeletal muscle cells from ischemia reperfusion injury
Scientific Reports (2021)
-
Myospryn deficiency leads to impaired cardiac structure and function and schizophrenia-associated symptoms
Cell and Tissue Research (2021)
-
How progressive cancer endangers the heart: an intriguing and underestimated problem
Cancer and Metastasis Reviews (2020)