Abstract
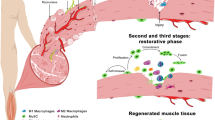
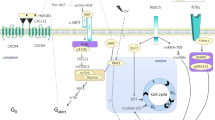
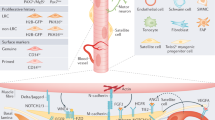
Skeletal muscle mass, function, and repair capacity all progressively decline with aging, restricting mobility, voluntary function, and quality of life. Skeletal muscle repair is facilitated by a population of dedicated muscle stem cells (MuSCs), also known as satellite cells, that reside in anatomically defined niches within muscle tissues. In adult tissues, MuSCs are retained in a quiescent state until they are primed to regenerate damaged muscle through cycles of self-renewal divisions. With aging, muscle tissue homeostasis is progressively disrupted and the ability of MuSCs to repair injured muscle markedly declines. Until recently, this decline has been largely attributed to extrinsic age-related alterations in the microenvironment to which MuSCs are exposed. However, as highlighted in this Perspective, recent reports show that MuSCs also progressively undergo cell-intrinsic alterations that profoundly affect stem cell regenerative function with aging. A more comprehensive understanding of the interplay of stem cell–intrinsic and extrinsic factors will set the stage for improving cell therapies capable of restoring tissue homeostasis and enhancing muscle repair in the aged.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Debbie Maizels/Nature Publishing Group

Debbie Maizels/Nature Publishing Group

Debbie Maizels/Nature Publishing Group

Debbie Maizels/Nature Publishing Group
Similar content being viewed by others
References
Buchman, T.G. The community of the self. Nature 420, 246–251 (2002).
Seale, P., Asakura, A. & Rudnicki, M.A. The potential of muscle stem cells. Dev. Cell 1, 333–342 (2001).
Cheung, T.H. & Rando, T.A. Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 14, 329–340 (2013).
Montarras, D., L'Honore, A. & Buckingham, M. Lying low but ready for action: the quiescent muscle satellite cell. FEBS J. 280, 4036–4050 (2013).
Bosnakovski, D. et al. Prospective isolation of skeletal muscle stem cells with a Pax7 reporter. Stem Cells 26, 3194–3204 (2008).
Cerletti, M. et al. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell 134, 37–47 (2008).
Kuang, S., Kuroda, K., Le Grand, F. & Rudnicki, M.A. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129, 999–1010 (2007).
Lepper, C. & Fan, C.M. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis 48, 424–436 (2010).
Montarras, D. et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science 309, 2064–2067 (2005).
Sacco, A., Doyonnas, R., Kraft, P., Vitorovic, S. & Blau, H.M. Self-renewal and expansion of single transplanted muscle stem cells. Nature 456, 502–506 (2008).
von Maltzahn, J., Jones, A.E., Parks, R.J. & Rudnicki, M.A. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc. Natl. Acad. Sci. USA 110, 16474–16479 (2013).
Günther, S. et al. Myf5-positive satellite cells contribute to Pax7-dependent long-term maintenance of adult muscle stem cells. Cell Stem Cell 13, 590–601 (2013).
Chakkalakal, J.V., Jones, K.M., Basson, M.A. & Brack, A.S. The aged niche disrupts muscle stem cell quiescence. Nature 490, 355–360 (2012).
Sambasivan, R. et al. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138, 3647–3656 (2011).
Gopinath, S.D., Webb, A.E., Brunet, A. & Rando, T.A. FOXO3 promotes quiescence in adult muscle stem cells during the process of self-renewal. Stem Cell Rep. 2, 414–426 (2014).
Rocheteau, P., Gayraud-Morel, B., Siegl-Cachedenier, I., Blasco, M.A. & Tajbakhsh, S. A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell 148, 112–125 (2012).
Fry, C.S. et al. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat. Med. 21, 76–80 (2015).
Dumont, N.A., Wang, Y.X. & Rudnicki, M.A. Intrinsic and extrinsic mechanisms regulating satellite cell function. Development 142, 1572–1581 (2015).
Gilbert, P.M. & Blau, H.M. Engineering a stem cell house into a home. Stem Cell Res. Ther. 2, 3 (2011).
Yin, H., Price, F. & Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 93, 23–67 (2013).
Cornelison, D.D., Filla, M.S., Stanley, H.M., Rapraeger, A.C. & Olwin, B.B. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev. Biol. 239, 79–94 (2001).
Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 9, 493–495 (1961).
Kuang, S., Gillespie, M.A. & Rudnicki, M.A. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell 2, 22–31 (2008).
Fukada, S. et al. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells 25, 2448–2459 (2007).
Price, F.D. et al. Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat. Med. 20, 1174–1181 (2014).
Nishijo, K. et al. Biomarker system for studying muscle, stem cells, and cancer in vivo. FASEB J. 23, 2681–2690 (2009).
Tierney, M.T. et al. STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat. Med. 20, 1182–1186 (2014).
Keefe, A.C. et al. Muscle stem cells contribute to myofibres in sedentary adult mice. Nat. Commun. 6, 7087 (2015).
Cosgrove, B.D. et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat. Med. 20, 255–264 (2014).
Cosgrove, B.D., Sacco, A., Gilbert, P.M. & Blau, H.M. A home away from home: challenges and opportunities in engineering in vitro muscle satellite cell niches. Differentiation 78, 185–194 (2009).
Gilbert, P.M. et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329, 1078–1081 (2010).
Carlson, B.M. & Faulkner, J.A. Muscle transplantation between young and old rats: age of host determines recovery. Am. J. Physiol. 256, C1262–C1266 (1989).
Carlson, B.M., Dedkov, E.I., Borisov, A.B. & Faulkner, J.A. Skeletal muscle regeneration in very old rats. J. Gerontol. A Biol. Sci. Med. Sci. 56, B224–B233 (2001).
Brack, A.S. et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317, 807–810 (2007).
Conboy, I.M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 (2005).
Johansson, C.B. et al. Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat. Cell Biol. 10, 575–583 (2008).
Brack, A.S., Conboy, I.M., Conboy, M.J., Shen, J. & Rando, T.A. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell 2, 50–59 (2008).
Conboy, I.M., Conboy, M.J., Smythe, G.M. & Rando, T.A. Notch-mediated restoration of regenerative potential to aged muscle. Science 302, 1575–1577 (2003).
Bjornson, C.R. et al. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells 30, 232–242 (2012).
Mourikis, P. et al. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells 30, 243–252 (2012).
Elabd, C. et al. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat. Commun. 5, 4082 (2014).
Sinha, M. et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science 344, 649–652 (2014).
Egerman, M.A. et al. GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab. 22, 164–174 (2015).
Loffredo, F.S. et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 153, 828–839 (2013).
Nakashima, M., Toyono, T., Akamine, A. & Joyner, A. Expression of growth/differentiation factor 11, a new member of the BMP/TGFβ superfamily during mouse embryogenesis. Mech. Dev. 80, 185–189 (1999).
Lee, S.J. Regulation of muscle mass by myostatin. Annu. Rev. Cell Dev. Biol. 20, 61–86 (2004).
Lee, S.J. et al. Regulation of muscle mass by follistatin and activins. Mol. Endocrinol. 24, 1998–2008 (2010).
Lee, Y.S. & Lee, S.J. Regulation of GDF-11 and myostatin activity by GASP-1 and GASP-2. Proc. Natl. Acad. Sci. USA 110, E3713–E3722 (2013).
McPherron, A.C. & Lee, S.J. Double muscling in cattle due to mutations in the myostatin gene. Proc. Natl. Acad. Sci. USA 94, 12457–12461 (1997).
Kambadur, R., Sharma, M., Smith, T.P. & Bass, J.J. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res. 7, 910–916 (1997).
McPherron, A.C., Lawler, A.M. & Lee, S.J. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat. Genet. 22, 260–264 (1999).
Brack, A.S. & Rando, T.A. Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev. 3, 226–237 (2007).
García-Prat, L., Sousa-Victor, P. & Munoz-Canoves, P. Functional dysregulation of stem cells during aging: a focus on skeletal muscle stem cells. FEBS J. 280, 4051–4062 (2013).
Notta, F. et al. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science 333, 218–221 (2011).
Bernet, J.D. et al. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat. Med. 20, 265–271 (2014).
Sousa-Victor, P. et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 506, 316–321 (2014).
Palacios, D. et al. TNF/p38alpha/polycomb signaling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell 7, 455–469 (2010).
Troy, A. et al. Coordination of satellite cell activation and self-renewal by Par-complex-dependent asymmetric activation of p38alpha/beta MAPK. Cell Stem Cell 11, 541–553 (2012).
Baker, D.J. et al. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nat. Cell Biol. 10, 825–836 (2008).
Baker, D.J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).
Hall, J.K., Banks, G.B., Chamberlain, J.S. & Olwin, B.B. Prevention of muscle aging by myofiber-associated satellite cell transplantation. Sci. Transl. Med. 2, 57ra83 (2010).
Chakkalakal, J.V. et al. Early forming label-retaining muscle stem cells require p27kip1 for maintenance of the primitive state. Development 141, 1649–1659 (2014).
Conboy, M.J., Karasov, A.O. & Rando, T.A. High incidence of non-random template strand segregation and asymmetric fate determination in dividing stem cells and their progeny. PLoS Biol. 5, e102 (2007).
Shinin, V., Gayraud-Morel, B., Gomes, D. & Tajbakhsh, S. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat. Cell Biol. 8, 677–687 (2006).
Bouché, M., Munoz-Canoves, P., Rossi, F. & Coletti, D. Inflammation in muscle repair, aging, and myopathies. Biomed Res. Int. 2014, 821950 (2014).
Tidball, J.G. Mechanisms of muscle injury, repair, and regeneration. Compr. Physiol. 1, 2029–2062 (2011).
Bosurgi, L., Manfredi, A.A. & Rovere-Querini, P. Macrophages in injured skeletal muscle: a perpetuum mobile causing and limiting fibrosis, prompting or restricting resolution and regeneration. Front. Immunol. 2, 62 (2011).
Chazaud, B. Macrophages: supportive cells for tissue repair and regeneration. Immunobiology 219, 172–178 (2014).
Arnold, L. et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069 (2007).
Cheng, M., Nguyen, M.H., Fantuzzi, G. & Koh, T.J. Endogenous interferon-gamma is required for efficient skeletal muscle regeneration. Am. J. Physiol. Cell Physiol. 294, C1183–C1191 (2008).
Perdiguero, E. et al. p38/MKP-1-regulated AKT coordinates macrophage transitions and resolution of inflammation during tissue repair. J. Cell Biol. 195, 307–322 (2011).
Collins, C.A., Zammit, P.S., Ruiz, A.P., Morgan, J.E. & Partridge, T.A. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells 25, 885–894 (2007).
Coppé, J.P. et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–2868 (2008).
Demaria, M. et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31, 722–733 (2014).
Joe, A.W. et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 12, 153–163 (2010).
Uezumi, A., Fukada, S., Yamamoto, N., Takeda, S. & Tsuchida, K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 12, 143–152 (2010).
Pretheeban, T., Lemos, D.R., Paylor, B., Zhang, R.H. & Rossi, F.M. Role of stem/progenitor cells in reparative disorders. Fibrogenesis Tissue Repair 5, 20 (2012).
Heredia, J.E. et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 153, 376–388 (2013).
Lemos, D.R. et al. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat. Med. 21, 786–794 (2015).
Murphy, M.M., Lawson, J.A., Mathew, S.J., Hutcheson, D.A. & Kardon, G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138, 3625–3637 (2011).
Rodeheffer, M.S. Tipping the scale: muscle versus fat. Nat. Cell Biol. 12, 102–104 (2010).
Serrano, A.L. et al. Cellular and molecular mechanisms regulating fibrosis in skeletal muscle repair and disease. Curr. Top. Dev. Biol. 96, 167–201 (2011).
Thorsteinsdóttir, S., Deries, M., Cachaco, A.S. & Bajanca, F. The extracellular matrix dimension of skeletal muscle development. Dev. Biol. 354, 191–207 (2011).
Kothari, P. et al. IL-6-mediated induction of matrix metalloproteinase-9 is modulated by JAK-dependent IL-10 expression in macrophages. J. Immunol. 192, 349–357 (2014).
Yu, Q. & Stamenkovic, I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 14, 163–176 (2000).
Philippou, A., Maridaki, M. & Koutsilieris, M. The role of urokinase-type plasminogen activator (uPA) and transforming growth factor beta 1 (TGFβ1) in muscle regeneration. In Vivo 22, 735–750 (2008).
Snow, M.H. The effects of aging on satellite cells in skeletal muscles of mice and rats. Cell Tissue Res. 185, 399–408 (1977).
Kovanen, V., Suominen, H., Risteli, J. & Risteli, L. Type IV collagen and laminin in slow and fast skeletal muscle in rats–effects of age and life-time endurance training. Coll. Relat. Res. 8, 145–153 (1988).
Alexakis, C., Partridge, T. & Bou-Gharios, G. Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am. J. Physiol. Cell Physiol. 293, C661–C669 (2007).
Scimè, A. et al. Transcriptional profiling of skeletal muscle reveals factors that are necessary to maintain satellite cell integrity during ageing. Mech. Ageing Dev. 131, 9–20 (2010).
Paliwal, P., Pishesha, N., Wijaya, D. & Conboy, I.M. Age dependent increase in the levels of osteopontin inhibits skeletal muscle regeneration. Aging (Albany, NY) 4, 553–566 (2012).
Rosant, C., Nagel, M.D. & Perot, C. Aging affects passive stiffness and spindle function of the rat soleus muscle. Exp. Gerontol. 42, 301–308 (2007).
Gao, Y., Kostrominova, T.Y., Faulkner, J.A. & Wineman, A.S. Age-related changes in the mechanical properties of the epimysium in skeletal muscles of rats. J. Biomech. 41, 465–469 (2008).
Engler, A.J. et al. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 166, 877–887 (2004).
Boonen, K.J., Rosaria-Chak, K.Y., Baaijens, F.P., van der Schaft, D.W. & Post, M.J. Essential environmental cues from the satellite cell niche: optimizing proliferation and differentiation. Am. J. Physiol. Cell Physiol. 296, C1338–C1345 (2009).
Liu, H., Niu, A., Chen, S.E. & Li, Y.P. Beta3-integrin mediates satellite cell differentiation in regenerating mouse muscle. FASEB J. 25, 1914–1921 (2011).
Wang, H.V. et al. Integrin-linked kinase stabilizes myotendinous junctions and protects muscle from stress-induced damage. J. Cell Biol. 180, 1037–1049 (2008).
Pisconti, A., Cornelison, D.D., Olguin, H.C., Antwine, T.L. & Olwin, B.B. Syndecan-3 and Notch cooperate in regulating adult myogenesis. J. Cell Biol. 190, 427–441 (2010).
Urciuolo, A. et al. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat. Commun. 4, 1964 (2013).
Bentzinger, C.F. et al. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell 12, 75–87 (2013).
Engler, A.J., Sen, S., Sweeney, H.L. & Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Halder, G., Dupont, S. & Piccolo, S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat. Rev. Mol. Cell Biol. 13, 591–600 (2012).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Pelissier, F.A. et al. Age-related dysfunction in mechanotransduction impairs differentiation of human mammary epithelial progenitors. Cell Rep 7, 1926–1939 (2014).
Lechler, T. & Fuchs, E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437, 275–280 (2005).
Quyn, A.J. et al. Spindle orientation bias in gut epithelial stem cell compartments is lost in precancerous tissue. Cell Stem Cell 6, 175–181 (2010).
Liu, L. et al. Chromatin modifications as determinants of muscle stem cell quiescence and chronological aging. Cell Rep 4, 189–204 (2013).
McKinnell, I.W. et al. Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nat. Cell Biol. 10, 77–84 (2008).
Paylor, B., Natarajan, A., Zhang, R.H. & Rossi, F. Nonmyogenic cells in skeletal muscle regeneration. Curr. Top. Dev. Biol. 96, 139–165 (2011).
Bentzinger, C.F., Wang, Y.X., Dumont, N.A. & Rudnicki, M.A. Cellular dynamics in the muscle satellite cell niche. EMBO Rep. 14, 1062–1072 (2013).
Bonfanti, C. et al. PW1/Peg3 expression regluates key properties that determine mesoangioblast stem cell competence. Nat. Commun. 6, 6364 (2015).
Campisi, J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522 (2005).
Discher, D.E., Mooney, D.J. & Zandstra, P.W. Growth factors, matrices, and forces combine and control stem cells. Science 324, 1673–1677 (2009).
Paszek, M.J. et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005).
Humphrey, J.D., Dufresne, E.R. & Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 15, 802–812 (2014).
Acknowledgements
We apologize to those investigators whose important work we were unable to cite or describe in depth owing to the limited scope and space constraints of this Perspective. We are grateful for support from Muscular Dystrophy Association grant 217821 (A.T.V.H.); support from the US National Institutes of Health (NIH) grant R00AG042491 (B.D.C.); and The Baxter Foundation, California Institute for Regenerative Medicine (CIRM) grants RB5-07469 and TR3-05501, and NIH grants AR063963, AG020961, AG044815, and NS089533 (H.M.B.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Blau, H., Cosgrove, B. & Ho, A. The central role of muscle stem cells in regenerative failure with aging. Nat Med 21, 854–862 (2015). https://doi.org/10.1038/nm.3918
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3918
This article is cited by
-
MuSCs and IPCs: roles in skeletal muscle homeostasis, aging and injury
Cellular and Molecular Life Sciences (2024)
-
Insight into muscle stem cell regeneration and mechanobiology
Stem Cell Research & Therapy (2023)
-
Machine learning-based classification of dual fluorescence signals reveals muscle stem cell fate transitions in response to regenerative niche factors
npj Regenerative Medicine (2023)
-
Clinical-grade human umbilical cord-derived mesenchymal stem cells improved skeletal muscle dysfunction in age-associated sarcopenia mice
Cell Death & Disease (2023)
-
Reduced smooth muscle-fibroblasts transformation potentially decreases intestinal wound healing and colitis-associated cancer in ageing mice
Signal Transduction and Targeted Therapy (2023)