Abstract
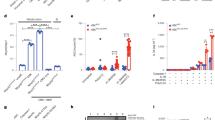
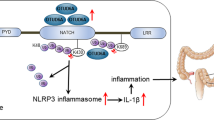
The inflammasome activates caspase-1 and the release of interleukin-1β (IL-1β) and IL-18, and several inflammasomes protect against intestinal inflammation and colitis-associated colon cancer (CAC) in animal models. The absent in melanoma 2 (AIM2) inflammasome is activated by double-stranded DNA, and AIM2 expression is reduced in several types of cancer, but the mechanism by which AIM2 restricts tumor growth remains unclear. We found that Aim2-deficient mice had greater tumor load than Asc-deficient mice in the azoxymethane/dextran sodium sulfate (AOM/DSS) model of colorectal cancer. Tumor burden was also higher in Aim2−/−/ApcMin/+ than in APCMin/+ mice. The effects of AIM2 on CAC were independent of inflammasome activation and IL-1β and were primarily mediated by a non–bone marrow source of AIM2. In resting cells, AIM2 physically interacted with and limited activation of DNA-dependent protein kinase (DNA-PK), a PI3K-related family member that promotes Akt phosphorylation, whereas loss of AIM2 promoted DNA-PK–mediated Akt activation. AIM2 reduced Akt activation and tumor burden in colorectal cancer models, while an Akt inhibitor reduced tumor load in Aim2−/− mice. These findings suggest that Akt inhibitors could be used to treat AIM2-deficient human cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Weir, H.K. et al. Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J. Natl. Cancer Inst. 95, 1276–1299 (2003).
Karin, M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb. Perspect. Biol. 1, a000141 (2009).
Hugot, J.P. et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411, 599–603 (2001).
Ogura, Y. et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411, 603–606 (2001).
Dupaul-Chicoine, J. et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity 32, 367–378 (2010).
Zaki, M.H. et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32, 379–391 (2010).
Allen, I.C. et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 207, 1045–1056 (2010).
Zaki, M.H., Vogel, P., Body-Malapel, M., Lamkanfi, M. & Kanneganti, T.D. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J. Immunol. 185, 4912–4920 (2010).
Salcedo, R. et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J. Exp. Med. 207, 1625–1636 (2010).
Chen, G.Y., Liu, M., Wang, F., Bertin, J. & Nunez, G. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J. Immunol. 186, 7187–7194 (2011).
Elinav, E. et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757 (2011).
Hu, B. et al. Microbiota-induced activation of epithelial IL-6 signaling links inflammasome-driven inflammation with transmissible cancer. Proc. Natl. Acad. Sci. USA 110, 9862–9867 (2013).
Normand, S. et al. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc. Natl. Acad. Sci. USA 108, 9601–9606 (2011).
Hu, B. et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc. Natl. Acad. Sci. USA 107, 21635–21640 (2010).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Fernandes-Alnemri, T., Yu, J.W., Datta, P., Wu, J. & Alnemri, E.S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 (2009).
DeYoung, K.L. et al. Cloning a novel member of the human interferon-inducible gene family associated with control of tumorigenicity in a model of human melanoma. Oncogene 15, 453–457 (1997).
Woerner, S.M. et al. Microsatellite instability of selective target genes in HNPCC-associated colon adenomas. Oncogene 24, 2525–2535 (2005).
Woerner, S.M. et al. The putative tumor suppressor AIM2 is frequently affected by different genetic alterations in microsatellite unstable colon cancers. Genes Chromosom. Cancer 46, 1080–1089 (2007).
Dihlmann, S. et al. Lack of Absent in Melanoma 2 (AIM2) expression in tumor cells is closely associated with poor survival in colorectal cancer patients. Int. J. Cancer 135, 2387–2396 (2014).
Choubey, D., Walter, S., Geng, Y. & Xin, H. Cytoplasmic localization of the interferon-inducible protein that is encoded by the AIM2 (absent in melanoma) gene from the 200-gene family. FEBS Lett. 474, 38–42 (2000).
Chen, I.F. et al. AIM2 suppresses human breast cancer cell proliferation in vitro and mammary tumor growth in a mouse model. Mol. Cancer Ther. 5, 1–7 (2006).
Patsos, G., Germann, A., Gebert, J. & Dihlmann, S. Restoration of absent in melanoma 2 (AIM2) induces G2/M cell cycle arrest and promotes invasion of colorectal cancer cells. Int. J. Cancer 126, 1838–1849 (2010).
Neufert, C., Becker, C. & Neurath, M.F. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat. Protoc. 2, 1998–2004 (2007).
Powrie, F., Leach, M.W., Mauze, S., Caddle, L.B. & Coffman, R.L. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 5, 1461–1471 (1993).
Dinarello, C.A. Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev. 29, 317–329 (2010).
Moser, A.R., Pitot, H.C. & Dove, W.F. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247, 322–324 (1990).
Rakoff-Nahoum, S. & Medzhitov, R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science 317, 124–127 (2007).
Lee, J., Li, L., Gretz, N., Gebert, J. & Dihlmann, S. Absent in Melanoma 2 (AIM2) is an important mediator of interferon-dependent and -independent HLA-DRA and HLA-DRB gene expression in colorectal cancers. Oncogene 31, 1242–1253 (2012).
Dhillon, A.S., Hagan, S., Rath, O. & Kolch, W. MAP kinase signalling pathways in cancer. Oncogene 26, 3279–3290 (2007).
Grivennikov, S.I. & Karin, M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 21, 11–19 (2010).
Parsons, D.W. et al. Colorectal cancer: mutations in a signalling pathway. Nature 436, 792 (2005).
Stephens, L. et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 279, 710–714 (1998).
Sarbassov, D.D., Guertin, D.A., Ali, S.M. & Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 (2005).
Feng, J., Park, J., Cron, P., Hess, D. & Hemmings, B.A. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J. Biol. Chem. 279, 41189–41196 (2004).
Li, Y. et al. Protein phosphatase 2A and DNA-dependent protein kinase are involved in mediating rapamycin-induced Akt phosphorylation. J. Biol. Chem. 288, 13215–13224 (2013).
Lu, D., Huang, J. & Basu, A. Protein kinase Cε activates protein kinase B/Akt via DNA-PK to protect against tumor necrosis factor-α-induced cell death. J. Biol. Chem. 281, 22799–22807 (2006).
Stambolic, V. et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95, 29–39 (1998).
Li, Z. et al. Effect of adenovirus-mediated PTEN gene on ulcerative colitis-associated colorectal cancer. Int. J. Colorectal Dis. 28, 1107–1115 (2013).
Ma, J. et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J. Natl. Cancer Inst. 91, 620–625 (1999).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Wang, Y. et al. Capture and 3D culture of colonic crypts and colonoids in a microarray platform. Lab Chip 13, 4625–4634 (2013).
Miyoshi, H. & Stappenbeck, T.S. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat. Protoc. 8, 2471–2482 (2013).
Yang, L. et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 64, 4394–4399 (2004).
Roberts, S.A. et al. Ku is a 5′-dRP/AP lyase that excises nucleotide damage near broken ends. Nature 464, 1214–1217 (2010).
Hosoi, Y. et al. Up-regulation of DNA-dependent protein kinase activity and Sp1 in colorectal cancer. Int. J. Oncol. 25, 461–468 (2004).
An, J. et al. DNA-dependent protein kinase catalytic subunit modulates the stability of c-Myc oncoprotein. Mol. Cancer 7, 32 (2008).
Cooper, A. et al. HIV-1 causes CD4 cell death through DNA-dependent protein kinase during viral integration. Nature 498, 376–379 (2013).
Mariathasan, S. et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 (2004).
Ostanin, D.V. et al. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G135–G146 (2009).
Petrucelli, A., Umashankar, M., Zagallo, P., Rak, M. & Goodrum, F. Interactions between proteins encoded within the human cytomegalovirus UL133-UL138 locus. J. Virol. 86, 8653–8662 (2012).
Acknowledgements
We thank E.H. Guthrie for technical assistance and I.C. Allen from the Department of Biomedical Sciences and Pathobiology at Virginia Tech for technical advice regarding the CAC model. P.K. Lund and S. Ding from the Department of Cell Biology and Physiology at UNC for their helpful discussions and technical advice pertaining to the ApcMin/+ model of spontaneous intestinal cancer; the Center for Gastrointestinal Biology and Disease (CGIBD) at UNC for providing core and technical support; and the Department of Microbiology and Immunology Flow Cytometry Core Facility at UNC for providing core and technical support. This work was supported by National Institute of Health (NIH) grants RO1-CA156330 (National Cancer Institute), U19-AI067798 (National Institute of Allergy and Infectious Disease (NIAID)), R37-AI029564 (NIAID) and P01 DK094779 (National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)) (J.P.-Y.T.). J.E.W. was supported in part by the NIH postdoctoral fellowship F32-K088417-01 (NIDDK) and in part by the American Cancer Society, PF-13-401-01-TBE. This work was also supported by the NIH grants R01 EY024556 (National Eye Institute) (N.L.A. and Y.W.) and R15 DK098754 (NIDDK) (B.K.D.).
Author information
Authors and Affiliations
Contributions
J.E.W. and A.S.P. contributed equally to this manuscript. J.E.W., A.S.P. and J.P.-Y.T designed the experiments and wrote the manuscript with input from B.K.D. and D.A.R. J.E.W., A.S.P. and L.C. performed most of the analyses. A.S.P. generated the AIM2 constructs and performed lentivirus transductions. W.-C.C. assisted in generating primary fibroblasts. M.S. performed the qPCR cytokine experiments. A.A.K, A.D.T. and W.J.B. assisted in the CAC and APCmin/+ animal studies. A.B.R. performed the histopathological scoring. L.C. performed the clinical scoring. Y.W. and N.L.A. developed and generated the primary organoid cultures. B.R.B. and Y.O. performed the flow cytometric analysis. M.M. and C.J. performed and oversaw the mini-endoscopy. All contributing authors have agreed to submission of this manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
Supplementary Figures 1–10 (PDF 17247 kb)
Rights and permissions
About this article
Cite this article
Wilson, J., Petrucelli, A., Chen, L. et al. Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nat Med 21, 906–913 (2015). https://doi.org/10.1038/nm.3908
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3908
This article is cited by
-
Cytosolic DNA sensors in neurodegenerative diseases: from physiological defenders to pathological culprits
EMBO Molecular Medicine (2024)
-
A probiotic bi-functional peptidoglycan hydrolase sheds NOD2 ligands to regulate gut homeostasis in female mice
Nature Communications (2023)
-
Context-dependent functions of pattern recognition receptors in cancer
Nature Reviews Cancer (2022)
-
AIM2 deficiency in B cells ameliorates systemic lupus erythematosus by regulating Blimp-1–Bcl-6 axis-mediated B-cell differentiation
Signal Transduction and Targeted Therapy (2021)
-
Absent in melanoma 2 suppresses gastric cancer cell proliferation and migration via inactivation of AKT signaling pathway
Scientific Reports (2021)