Abstract
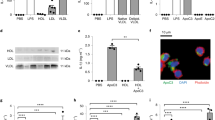
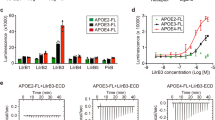
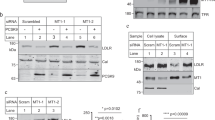
Interleukin-18 (IL18) participates in atherogenesis through several putative mechanisms1,2. Interruption of IL18 action reduces atherosclerosis in mice3,4. Here, we show that absence of the IL18 receptor (IL18r) does not affect atherosclerosis in apolipoprotein E–deficient (Apoe−/−) mice, nor does it affect IL18 cell surface binding to or signaling in endothelial cells. As identified initially by co-immunoprecipitation with IL18, we found that IL18 interacts with the Na-Cl co-transporter (NCC; also known as SLC12A3), a 12-transmembrane-domain ion transporter protein preferentially expressed in the kidney5. NCC is expressed in atherosclerotic lesions, where it colocalizes with IL18r. In Apoe−/− mice, combined deficiency of IL18r and NCC, but not single deficiency of either protein, protects mice from atherosclerosis. Peritoneal macrophages from Apoe−/− mice or from Apoe−/− mice lacking IL18r or NCC show IL18 binding and induction of cell signaling and cytokine and chemokine expression, but macrophages from Apoe−/− mice with combined deficiency of IL18r and NCC have a blunted response. An interaction between NCC and IL18r on macrophages was detected by co-immunoprecipitation. IL18 binds to the cell surface of NCC-transfected COS-7 cells, which do not express IL18r, and induces cell signaling and cytokine expression. This study identifies NCC as an IL18-binding protein that collaborates with IL18r in cell signaling, inflammatory molecule expression, and experimental atherogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dinarello, C.A. IL-18: A TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J. Allergy Clin. Immunol. 103, 11–24 (1999).
Yoshimoto, T. et al. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J. Immunol. 161, 3400–3407 (1998).
Elhage, R. et al. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc. Res. 59, 234–240 (2003).
Mallat, Z. et al. Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ. Res. 89, E41–E45 (2001).
Gamba, G. et al. Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J. Biol. Chem. 269, 17713–17722 (1994).
Okamura, H., Kashiwamura, S., Tsutsui, H., Yoshimoto, T. & Nakanishi, K. Regulation of interferon-gamma production by IL-12 and IL-18. Curr. Opin. Immunol. 10, 259–264 (1998).
Konishi, H. et al. IL-18 contributes to the spontaneous development of atopic dermatitis-like inflammatory skin lesion independently of IgE/stat6 under specific pathogen-free conditions. Proc. Natl. Acad. Sci. USA 99, 11340–11345 (2002).
French, A.R., Holroyd, E.B., Yang, L., Kim, S. & Yokoyama, W.M. IL-18 acts synergistically with IL-15 in stimulating natural killer cell proliferation. Cytokine 35, 229–234 (2006).
Netea, M.G. et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat. Med. 12, 650–656 (2006).
Sugiyama, M. et al. Deletion of IL-18 receptor ameliorates renal injury in bovine serum albumin-induced glomerulonephritis. Clin. Immunol. 128, 103–108 (2008).
Gutcher, I., Urich, E., Wolter, K., Prinz, M. & Becher, B. Interleukin 18-independent engagement of interleukin 18 receptor-alpha is required for autoimmune inflammation. Nat. Immunol. 7, 946–953 (2006).
Mallat, Z. et al. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation 104, 1598–1603 (2001).
Gerdes, N. et al. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J. Exp. Med. 195, 245–257 (2002).
Whitman, S.C., Ravisankar, P. & Daugherty, A. Interleukin-18 enhances atherosclerosis in apolipoprotein E(−/−) mice through release of interferon-gamma. Circ. Res. 90, E34–E38 (2002).
Tenger, C., Sundborger, A., Jawien, J. & Zhou, X. IL-18 accelerates atherosclerosis accompanied by elevation of IFN-gamma and CXCL16 expression independently of T cells. Arterioscler. Thromb. Vasc. Biol. 25, 791–796 (2005).
Torigoe, K. et al. Purification and characterization of the human interleukin-18 receptor. J. Biol. Chem. 272, 25737–25742 (1997).
Hoshino, K. et al. Cutting edge: generation of IL-18 receptor-deficient mice: evidence for IL-1 receptor-related protein as an essential IL-18 binding receptor. J. Immunol. 162, 5041–5044 (1999).
Kunchaparty, S. et al. Defective processing and expression of thiazide-sensitive Na-Cl cotransporter as a cause of Gitelman's syndrome. Am. J. Physiol. 277, F643–F649 (1999).
Gamba, G. et al. Primary structure and functional expression of a cDNA encoding the thiazide-sensitive, electroneutral sodium-chloride cotransporter. Proc. Natl. Acad. Sci. USA 90, 2749–2753 (1993).
Schultheis, P.J. et al. Phenotype resembling Gitelman's syndrome in mice lacking the apical Na+-Cl− cotransporter of the distal convoluted tubule. J. Biol. Chem. 273, 29150–29155 (1998).
Gjata, M., Tase, M., Gjata, A. & Gjergji, Zh. Gitelman's syndrome (familial hypokalemia-hypomagnesemia). Hippokratia. 11, 150–153 (2007).
Morris, R.G., Hoorn, E.J. & Knepper, M.A. Hypokalemia in a mouse model of Gitelman's syndrome. Am. J. Physiol. Renal Physiol. 290, F1416–F1420 (2006).
Tzanakis, I. et al. Intra- and extracellular magnesium levels and atheromatosis in haemodialysis patients. Magnes. Res. 17, 102–108 (2004).
Hulthe, J. et al. Plasma interleukin (IL)-18 concentrations is elevated in patients with previous myocardial infarction and related to severity of coronary atherosclerosis independently of C-reactive protein and IL-6. Atherosclerosis 188, 450–454 (2006).
Blankenberg, S. et al. Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation 106, 24–30 (2002).
Lauwerys, B.R., Renauld, J.C. & Houssiau, F.A. Synergistic proliferation and activation of natural killer cells by interleukin 12 and interleukin 18. Cytokine 11, 822–830 (1999).
Brunet, A. et al. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 18, 664–674 (1999).
Ono, K. & Han, J. The p38 signal transduction pathway: activation and function. Cell. Signal. 12, 1–13 (2000).
Hossain Khan, M.Z. et al. Phosphorylation of Na-Cl cotransporter by OSR1 and SPAK kinases regulates its ubiquitination. Biochem. Biophys. Res. Commun. 425, 456–461 (2012).
Yang, S.S. et al. Generation and analysis of the thiazide-sensitive Na+-Cl− cotransporter (Ncc/Slc12a3) Ser707X knockin mouse as a model of Gitelman syndrome. Hum. Mutat. 31, 1304–1315 (2010).
Pacheco-Alvarez, D. et al. The Na+:Cl− cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J. Biol. Chem. 281, 28755–28763 (2006).
Rozansky, D.J. et al. Aldosterone mediates activation of the thiazide-sensitive Na-Cl cotransporter through an SGK1 and WNK4 signaling pathway. J. Clin. Invest. 119, 2601–2612 (2009).
Griffon, N., Jeanneteau, F., Prieur, F., Diaz, J. & Sokoloff, P. CLIC6, a member of the intracellular chloride channel family, interacts with dopamine D(2)-like receptors. Brain Res. Mol. Brain Res. 117, 47–57 (2003).
Heim, M.H. The Jak-STAT pathway: cytokine signalling from the receptor to the nucleus. J. Recept. Signal Transduct. Res. 19, 75–120 (1999).
De Jong, J.C. et al. Functional expression of mutations in the human NaCl cotransporter: evidence for impaired routing mechanisms in Gitelman's syndrome. J. Am. Soc. Nephrol. 13, 1442–1448 (2002).
Glaudemans, B. et al. Novel NCC mutants and functional analysis in a new cohort of patients with Gitelman syndrome. Eur. J. Hum. Genet. 20, 263–270 (2012).
Hoorn, E.J. et al. The calcineurin inhibitor tacrolimus activates the renal sodium chloride cotransporter to cause hypertension. Nat. Med. 17, 1304–1309 (2011).
Reilly, R.F. & Ellison, D.H. Mammalian distal tubule: physiology, pathophysiology, and molecular anatomy. Physiol. Rev. 80, 277–313 (2000).
Miyauchi, K., Takiyama, Y., Honjyo, J., Tateno, M. & Haneda, M. Upregulated IL-18 expression in type 2 diabetic subjects with nephropathy: TGF-beta1 enhanced IL-18 expression in human renal proximal tubular epithelial cells. Diabetes Res. Clin. Pract. 83, 190–199 (2009).
Kinoshita, K. et al. Blockade of IL-18 receptor signaling delays the onset of autoimmune disease in MRL-Faslpr mice. J. Immunol. 173, 5312–5318 (2004).
Sukhova, G.K. et al. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J. Clin. Invest. 111, 897–906 (2003).
Mach, F., Schönbeck, U., Sukhova, G.K., Atkinson, E. & Libby, P. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature 394, 200–203 (1998).
Zhang, M.Z. et al. Role of blood pressure and the renin-angiotensin system in development of diabetic nephropathy (DN) in eNOS−/−db/db mice. Am. J. Physiol. Renal Physiol. 302, F433–F438 (2012).
Bostanjoglo, M. et al. 11Beta-hydroxysteroid dehydrogenase, mineralocorticoid receptor, and thiazide-sensitive Na-Cl cotransporter expression by distal tubules. J. Am. Soc. Nephrol. 9, 1347–1358 (1998).
Zhang, J. et al. Regulation of endothelial cell adhesion molecule expression by mast cells, macrophages, and neutrophils. PLoS ONE 6, e14525 (2011).
Shi, G.P. et al. Cathepsin S required for normal MHC class II peptide loading and germinal center development. Immunity 10, 197–206 (1999).
Riveira-Munoz, E. et al. Transcriptional and functional analyses of SLC12A3 mutations: new clues for the pathogenesis of Gitelman syndrome. J. Am. Soc. Nephrol. 18, 1271–1283 (2007).
Markadieu, N. et al. A primary culture of distal convoluted tubules expressing functional thiazide-sensitive NaCl transport. Am. J. Physiol. Renal Physiol. 303, F886–F892 (2012).
Acknowledgements
This study is supported by grants from the US National Heart, Lung, and Blood Institute (HL60942, HL81090, HL88547 to G.-P.S.; HL34636, HL80472 to P.L.) and by an American Heart Association Established Investigator Award (0840118N to G.-P.S.).
Author information
Authors and Affiliations
Contributions
J.W. and C.S. performed most of the experiments. N.G. completed the original IL18 and IL18r mutant mouse analysis. C.L., M.L., M.A.S., A.H., Y.Z., H.C., J.Z., X.C. and Q.K. performed RT-PCR, lesion analysis, cell culture and plasma ELISA. J.L. helped with the NCC cDNA cloning. G.K.S. performed immunostaining. X.W.C., M.K., T.M. and P.L. helped with experimental design, writing and data interpretation. M.J. and G.E.S. provided the NCC mutant mice. S.R., C.-L.Y. and D.H.E. provided the NCC monoclonal antibody and performed the 293 cell experiments. S.J. and R.B. made the human NCC mutant constructs. P.J. measured plasma Mg and K. G.-P.S. designed and performed the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 18796 kb)
Rights and permissions
About this article
Cite this article
Wang, J., Sun, C., Gerdes, N. et al. Interleukin 18 function in atherosclerosis is mediated by the interleukin 18 receptor and the Na-Cl co-transporter. Nat Med 21, 820–826 (2015). https://doi.org/10.1038/nm.3890
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3890
This article is cited by
-
Biological and clinical roles of IL-18 in inflammatory diseases
Nature Reviews Rheumatology (2024)
-
Clonal hematopoiesis in cardiovascular disease and therapeutic implications
Nature Cardiovascular Research (2022)
-
Differential IL18 signaling via IL18 receptor and Na-Cl co-transporter discriminating thermogenesis and glucose metabolism regulation
Nature Communications (2022)
-
Adipokines, adiposity, and atherosclerosis
Cellular and Molecular Life Sciences (2022)
-
In Silico Development of Combinatorial Therapeutic Approaches Targeting Key Signaling Pathways in Metabolic Syndrome
Pharmaceutical Research (2022)