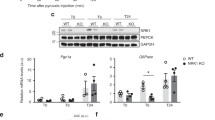
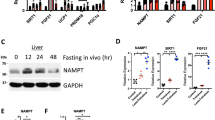
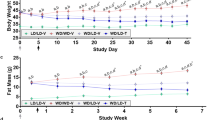
Abstract
Nicotinamide N-methyltransferase (Nnmt) methylates nicotinamide, a form of vitamin B3, to produce N1-methylnicotinamide (MNAM). Nnmt has emerged as a metabolic regulator in adipocytes, but its role in the liver, the tissue with the strongest Nnmt expression, is not known. In spite of its overall high expression, here we find that hepatic expression of Nnmt is highly variable and correlates with multiple metabolic parameters in mice and humans. Further, we find that suppression of hepatic Nnmt expression in vivo alters glucose and cholesterol metabolism and that the metabolic effects of Nnmt in the liver are mediated by its product MNAM. Supplementation of high-fat diet with MNAM decreases serum and liver cholesterol and liver triglycerides levels in mice. Mechanistically, increasing Nnmt expression or MNAM levels stabilizes sirtuin 1 protein, an effect that is required for their metabolic benefits. In summary, we describe here a novel regulatory pathway for vitamin B3 that could provide a new opportunity for metabolic disease therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Haigis, M.C. & Sinclair, D.A. Mammalian sirtuins: biological insights and disease relevance. Annu. Rev. Pathol. 5, 253–295 (2010).
Houtkooper, R.H., Cantó, C., Wanders, R.J. & Auwerx, J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr. Rev. 31, 194–223 (2010).
Bogan, K.L. & Brenner, C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu. Rev. Nutr. 28, 115–130 (2008).
Rodgers, J.T. et al. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434, 113–118 (2005).
Nemoto, S., Fergusson, M.M. & Finkel, T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science 306, 2105–2108 (2004).
Nemoto, S., Fergusson, M.M. & Finkel, T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J. Biol. Chem. 280, 16456–16460 (2005).
Liu, Y. et al. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature 456, 269–273 (2008).
Rodgers, J.T. & Puigserver, P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc. Natl. Acad. Sci. USA 104, 12861–12866 (2007).
Erion, D.M. et al. SirT1 knockdown in liver decreases basal hepatic glucose production and increases hepatic insulin responsiveness in diabetic rats. Proc. Natl. Acad. Sci. USA 106, 11288–11293 (2009).
Ponugoti, B. et al. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J. Biol. Chem. 285, 33959–33970 (2010).
Walker, A.K. et al. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 24, 1403–1417 (2010).
Li, Y. et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 13, 376–388 (2011).
Xu, F. et al. Lack of SIRT1 (mammalian Sirtuin 1) activity leads to liver steatosis in the SIRT1+/− mice: a role of lipid mobilization and inflammation. Endocrinology 151, 2504–2514 (2010).
Purushotham, A. et al. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 9, 327–338 (2009).
Wang, R.-H. et al. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J. Clin. Invest. (2011).
Bordone, L. et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 6, 759–767 (2007).
Banks, A.S. et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 8, 333–341 (2008).
Pfluger, P.T., Herranz, D., Velasco-Miguel, S., Serrano, M. & Tschöp, M.H. Sirt1 protects against high-fat diet–induced metabolic damage. Proc. Natl. Acad. Sci. USA 105, 9793–9798 (2008).
Yoshino, J., Mills, K.F., Yoon, M.J. & Imai, S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 14, 528–536 (2011).
Cantó, C. et al. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 15, 838–847 (2012).
Qiang, L. et al. Proatherogenic abnormalities of lipid metabolism in SirT1 transgenic mice are mediated through Creb deacetylation. Cell Metab. 14, 758–767 (2011).
Kim, J.-E., Chen, J. & Lou, Z. DBC1 is a negative regulator of SIRT1. Nature 451, 583–586 (2008).
Zhao, W. et al. Negative regulation of the deacetylase SIRT1 by DBC1. Nature 451, 587–590 (2008).
Kim, E.-J., Kho, J.-H., Kang, M.-R. & Um, S.-J. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol. Cell 28, 277–290 (2007).
Revollo, J.R., Grimm, A.A. & Imai, S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J. Biol. Chem. 279, 50754–50763 (2004).
Chalkiadaki, A. & Guarente, L. Sirtuins mediate mammalian metabolic responses to nutrient availability. Nat. Rev. Endocrinol. 8, 287–296 (2012).
Kraus, D. et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature 508, 258–262 (2014).
Kang-Lee, Y.A. et al. Metabolic effects of nicotinamide administration in rats. J. Nutr. 113, 215–221 (1983).
Scheller, T., Orgacka, H., Szumlanski, C.L. & Weinshilboum, R.M. Mouse liver nicotinamide N-methyltransferase pharmacogenetics: biochemical properties and variation in activity among inbred strains. Pharmacogenetics 6, 43–53 (1996).
Yan, L., Otterness, D.M., Craddock, T.L. & Weinshilboum, R.M. Mouse liver nicotinamide N-methyltransferase: cDNA cloning, expression, and nucleotide sequence polymorphisms. Biochem. Pharmacol. 54, 1139–1149 (1997).
Felsted, R.L. & Chaykin, S. N1-Methylnicotinamide oxidation in a number of mammals. J. Biol. Chem. 242, 1274–1279 (1967).
Ghazalpour, A. et al. Hybrid mouse diversity panel: a panel of inbred mouse strains suitable for analysis of complex genetic traits. Mamm. Genome 23, 680–692 (2012).
Ford, J., Ahmed, S., Allison, S., Jiang, M. & Milner, J. JNK2-dependent regulation of SIRT1 protein stability. Cell Cycle 7, 3091–3097 (2008).
Gao, Z. et al. Sirtuin 1 (SIRT1) protein degradation in response to persistent c-Jun N-terminal kinase 1 (JNK1) activation contributes to hepatic steatosis in obesity. J. Biol. Chem. 286, 22227–22234 (2011).
Dong, S. et al. The REGγ proteasome regulates hepatic lipid metabolism through inhibition of autophagy. Cell Metab. 18, 380–391 (2013).
Guex, N. & Peitsch, M.C. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18, 2714–2723 (1997).
Trott, O. & Olson, A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 (2010).
Sanner, M.F. Python: a programming language for software integration and development. J. Mol. Graph. Model. 17, 55–61 (1999).
Chlopicki, S. et al. 1-Methylnicotinamide (MNA), a primary metabolite of nicotinamide, exerts anti-thrombotic activity mediated by a cyclooxygenase-2/prostacyclin pathway. Br. J. Pharmacol. 152, 230–239 (2007).
Bryniarski, K., Biedron, R., Jakubowski, A., Chlopicki, S. & Marcinkiewicz, J. Anti-inflammatory effect of 1-methylnicotinamide in contact hypersensitivity to oxazolone in mice; involvement of prostacyclin. Eur. J. Pharmacol. 578, 332–338 (2008).
Bartusś, M. et al. 1-Methylnicotinamide (MNA) prevents endothelial dysfunction in hypertriglyceridemic and diabetic rats. Pharmacol. Rep. 60, 127–138 (2008).
Li, Y. et al. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J. 25, 1664–1679 (2011).
Kannt, A. et al. Association of nicotinamide-N-methyltransferase mRNA expression in human adipose tissue and the plasma concentration of its product, 1-methylnicotinamide, with insulin resistance. Diabetologia 58, 799–808 (2015).
Kennedy, A.R. et al. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am. J. Physiol. Endocrinol Metab. 292, E1724–E1739 (2007).
Estep, P.W., Warner, J.B. & Bulyk, M.L. Short-term calorie restriction in male mice feminizes gene expression and alters key regulators of conserved aging regulatory pathways. PLoS ONE 4, e5242 (2009).
Schadt, E.E. et al. Genetics of gene expression surveyed in maize, mouse and man. Nature 422, 297–302 (2003).
Ulanovskaya, O.A., Zuhl, A.M. & Cravatt, B.F. NNMT promotes epigenetic remodeling in cancer by creating a metabolic methylation sink. Nat. Chem. Biol. 9, 300–306 (2013).
Mato, J.M., Martínez-Chantar, M.L. & Lu, S.C. Methionine metabolism and liver disease. Annu. Rev. Nutr. 28, 273–293 (2008).
Chalkiadaki, A. & Guarente, L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 16, 180–188 (2012).
Peng, L. et al. Ubiquitinated sirtuin 1 (SIRT1) function is modulated during DNA damage-induced cell death and survival. J. Biol. Chem. 290, 8904–8912 (2015).
Schmeisser, K. et al. Role of sirtuins in lifespan regulation is linked to methylation of nicotinamide. Nat. Chem. Biol. 9, 693–700 (2013).
Li, X. et al. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol. Cell 28, 91–106 (2007).
Kemper, J.K. et al. FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab. 10, 392–404 (2009).
Kleiner, D.E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321 (2005).
Acknowledgements
We would like to thank F.F. Liu, D. Adams, A.M. Real and M.J. Lee for technical assistance and J.S. Flier for advice. We thank B.M. Spiegelman and P. Puigserver (Dana-Farber Cancer Center, Boston, MA) for the donation of the Sirt1 and FoxO1 adenoviruses, D.E. Cohen (Brigham and Women's Hospital, Boston, MA) for lipoprotein analysis and R. Ortiz (University Hospital of Girona, Spain) for the pathology evaluation of the human liver biopsies. This work was supported by grants from Takeda, the US National Institute of Health DK028082 (E.M.F.), DK083694 (P.P.), Boston Area Diabetes Endocrinology Research Center (BADERC) grant DK057521 (P.P.), and by grants FIS-PI11/00214 and FIS-PI12/02631 to J.M.F.R. from the Instituto de Salud Carlos III and Fondo Europeo de Desarrollo Regional (FEDER), Spain.
Author information
Authors and Affiliations
Contributions
S.H., Y.K., I.T. and P.P. designed, performed and interpreted experiments. X.W., D.P., Y.L. and J.M.A. performed experiments. E.M.-F. interpreted experiments. J.M.M.-N. and J.M.F.-R. performed and interpreted the human experiments. S.H., I.T., P.P. and E.M.-F. corrected the manuscript. P.P. conceived and managed the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 & Supplementary Tables 1–2 (PDF 2893 kb)
Rights and permissions
About this article
Cite this article
Hong, S., Moreno-Navarrete, J., Wei, X. et al. Nicotinamide N-methyltransferase regulates hepatic nutrient metabolism through Sirt1 protein stabilization. Nat Med 21, 887–894 (2015). https://doi.org/10.1038/nm.3882
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3882
This article is cited by
-
Multi-omics analysis of a fatty liver model using human hepatocyte chimeric mice
Scientific Reports (2024)
-
Control of NAD+ homeostasis by autophagic flux modulates mitochondrial and cardiac function
The EMBO Journal (2024)
-
Targeting protein modifications in metabolic diseases: molecular mechanisms and targeted therapies
Signal Transduction and Targeted Therapy (2023)
-
Stromal nicotinamide N-methyltransferase orchestrates the crosstalk between fibroblasts and tumour cells in oral squamous cell carcinoma: evidence from patient-derived assembled organoids
Oncogene (2023)
-
Ligand-based in silico identification and biological evaluation of potential inhibitors of nicotinamide N-methyltransferase
Molecular Diversity (2023)