Abstract
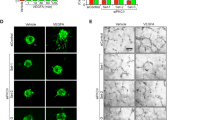
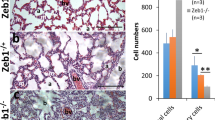
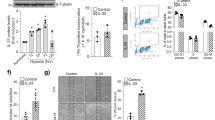
Ocular neovascular diseases are a leading cause of blindness. Vascular endothelial growth factor (VEGF) blockade improves vision, but not all individuals respond to anti-VEGF treatment, making additional means to prevent neovascularization necessary. Slit-family proteins (Slits) are ligands of Roundabout (Robo) receptors that repel developing axons in the nervous system. Robo1 expression is altered in ocular neovascular diseases, and previous in vitro studies have reported both pro- and anti-angiogenic effects of Slits. However, genetic evidence supporting a role for Slits in ocular neovascularization is lacking. Here we generated conditional knockout mice deficient in various Slit and Robo proteins and found that Slit2 potently and selectively promoted angiogenesis via Robo1 and Robo2 in mouse postnatal retina and in a model of ocular neovascular disease. Mechanistically, Slit2 acting through Robo1 and Robo2 promoted the migration of endothelial cells. These receptors are required for both Slit2- and VEGF-induced Rac1 activation and lamellipodia formation. Thus, Slit2 blockade could potentially be used therapeutically to inhibit angiogenesis in individuals with ocular neovascular disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ferrara, N. Vascular endothelial growth factor and age-related macular degeneration: from basic science to therapy. Nat. Med. 16, 1107–1111 (2010).
Miller, J.W., Le Couter, J., Strauss, E.C. & Ferrara, N. Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology 120, 106–114 (2013).
Lim, L.S., Mitchell, P., Seddon, J.M., Holz, F.G. & Wong, T.Y. Age-related macular degeneration. Lancet 379, 1728–1738 (2012).
Brose, K. et al. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 96, 795–806 (1999).
Kidd, T., Bland, K.S. & Goodman, C.S. Slit is the midline repellent for the robo receptor in Drosophila. Cell 96, 785–794 (1999).
Ypsilanti, A.R., Zagar, Y. & Chedotal, A. Moving away from the midline: new developments for Slit and Robo. Development 137, 1939–1952 (2010).
Koch, A.W. et al. Robo4 maintains vessel integrity and inhibits angiogenesis by interacting with UNC5B. Dev. Cell 20, 33–46 (2011).
Zelina, P. et al. Signaling switch of the axon guidance receptor Robo3 during vertebrate evolution. Neuron 84, 1258–1272 (2014).
Jones, C.A. et al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat. Med. 14, 448–453 (2008).
Jones, C.A. et al. Slit2-Robo4 signalling promotes vascular stability by blocking Arf6 activity. Nat. Cell Biol. 11, 1325–1331 (2009).
Huang, L. et al. Expression of Robo4 in the fibrovascular membranes from patients with proliferative diabetic retinopathy and its role in RF/6A and RPE cells. Mol. Vis. 15, 1057–1069 (2009).
Zhou, W. et al. The role of SLIT-ROBO signaling in proliferative diabetic retinopathy and retinal pigment epithelial cells. Mol. Vis. 17, 1526–1536 (2011).
Zhang, B. et al. Repulsive axon guidance molecule Slit3 is a novel angiogenic factor. Blood 114, 4300–4309 (2009).
Yang, X.M. et al. Slit-Robo signaling mediates lymphangiogenesis and promotes tumor lymphatic metastasis. Biochem. Biophys. Res. Commun. 396, 571–577 (2010).
Urbich, C. et al. HDAC5 is a repressor of angiogenesis and determines the angiogenic gene expression pattern of endothelial cells. Blood 113, 5669–5679 (2009).
Kaur, S. et al. Robo4 signaling in endothelial cells implies attraction guidance mechanisms. J. Biol. Chem. 281, 11347–11356 (2006).
Plump, A.S. et al. Slit1 and Slit2 cooperate to prevent premature midline crossing of retinal axons in the mouse visual system. Neuron 33, 219–232 (2002).
Fouquet, C. et al. Robo1 and Robo2 control the development of the lateral olfactory tract. J. Neurosci. 27, 3037–3045 (2007).
Morlot, C. et al. Structural insights into the Slit-Robo complex. Proc. Natl. Acad. Sci. USA 104, 14923–14928 (2007).
Guo, C., Yang, W. & Lobe, C.G. A Cre recombinase transgene with mosaic, widespread tamoxifen-inducible action. Genesis 32, 8–18 (2002).
Birdsey, G.M. et al. Transcription factor Erg regulates angiogenesis and endothelial apoptosis through VE-cadherin. Blood 111, 3498–3506 (2008).
Guijarro-Muñoz, I. et al. The axonal repellent Slit2 inhibits pericyte migration: potential implications in angiogenesis. Exp. Cell Res. 318, 371–378 (2012).
Sörensen, I., Adams, R.H. & Gossler, A. DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. Blood 113, 5680–5688 (2009).
Yoshioka, K. et al. Endothelial PI3K-C2α, a class II PI3K, has an essential role in angiogenesis and vascular barrier function. Nat. Med. 18, 1560–1569 (2012).
del Toro, R. et al. Identification and functional analysis of endothelial tip cell-enriched genes. Blood 116, 4025–4033 (2010).
Wang, B. et al. Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer Cell 4, 19–29 (2003).
Grieshammer, U. et al. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev. Cell 6, 709–717 (2004).
Lu, W. et al. Disruption of ROBO2 is associated with urinary tract anomalies and confers risk of vesicoureteral reflux. Am. J. Hum. Genet. 80, 616–632 (2007).
Long, H. et al. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron 42, 213–223 (2004).
Liu, D. et al. Neuronal chemorepellent Slit2 inhibits vascular smooth muscle cell migration by suppressing small GTPase Rac1 activation. Circ. Res. 98, 480–489 (2006).
Wang, Y. et al. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell 151, 1332–1344 (2012).
Adams, R.H. & Eichmann, A. Axon guidance molecules in vascular patterning. Cold Spring Harb. Perspect. Biol. 2, a001875 (2010).
Maisonpierre, P.C. et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277, 55–60 (1997).
Lu, X. et al. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature 432, 179–186 (2004).
Ridgway, J. et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature 444, 1083–1087 (2006).
Hellström, M. et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445, 776–780 (2007).
Larrivée, B. et al. ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev. Cell 22, 489–500 (2012).
Small, E.M., Sutherland, L.B., Rajagopalan, K.N., Wang, S. & Olson, E.N. MicroRNA-218 regulates vascular patterning by modulation of Slit-Robo signaling. Circ. Res. 107, 1336–1344 (2010).
Fish, J.E. et al. A Slit/miR-218/Robo regulatory loop is required during heart tube formation in zebrafish. Development 138, 1409–1419 (2011).
Ridley, A.J. et al. Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003).
Connor, K.M. et al. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat. Protoc. 4, 1565–1573 (2009).
Mehlen, P., Delloye-Bourgeois, C. & Chedotal, A. Novel roles for Slits and netrins: axon guidance cues as anticancer targets? Nat. Rev. Cancer 11, 188–197 (2011).
Benedito, R. et al. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature 484, 110–114 (2012).
Germain, S., Monnot, C., Muller, L. & Eichmann, A. Hypoxia-driven angiogenesis: role of tip cells and extracellular matrix scaffolding. Curr. Opin. Hematol. 17, 245–251 (2010).
Domyan, E.T. et al. Roundabout receptors are critical for foregut separation from the body wall. Dev. Cell 24, 52–63 (2013).
Marillat, V. et al. Spatiotemporal expression patterns of slit and robo genes in the rat brain. J. Comp. Neurol. 442, 130–155 (2002).
Nguyen-Ba-Charvet, K.T. et al. Multiple roles for slits in the control of cell migration in the rostral migratory stream. J. Neurosci. 24, 1497–1506 (2004).
Di Meglio, T. Nguyen-Ba-Charvet, K.T., Tessier-Lavigne, M., Sotelo, C. & Chédotal, A. Molecular mechanisms controlling midline crossing by precerebellar neurons. J. Neurosci. 28, 6285–6294 (2008).
Chauvet, S. et al. Gating of Sema3E/PlexinD1 signaling by neuropilin-1 switches axonal repulsion to attraction during brain development. Neuron 56, 807–822 (2007).
Xu, Y. et al. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J. Cell Biol. 188, 115–130 (2010).
Jones, E.A., Yuan, L., Breant, C., Watts, R.J. & Eichmann, A. Separating genetic and hemodynamic defects in neuropilin 1 knockout embryos. Development 135, 2479–2488 (2008).
Lanahan, A. et al. The neuropilin 1 cytoplasmic domain is required for VEGF-A-dependent arteriogenesis. Dev. Cell 25, 156–168 (2013).
Acknowledgements
This project was supported by grants from the Agence Nationale de la Recherche (ANR; ANR-11BSV102502 to A.C. and A.E.), the Fondation pour la Recherche Médicale (“Programme Équipe FRM” to A.C.; DEQ20120323700), Sanofi-Fovea (to A.C.), Fondation Leducq (Artemis Transatlantic Network of Excellence, to A.E.) and the Thome Foundation for Age-Related Macular Degeneration (to A.E.). It was performed in the frame of the LABEX LIFESENSES (reference ANR-10-LABX-65), supported by French state funds managed by the ANR within the Investissements d'Avenir program under reference ANR-11-IDEX-0004-02. We thank F. Sennlaub for help with OIR experiments; J. Sahel and T. Debeir for helpful suggestions; Y. Zagar, M. Belle and C. Dominici for technical help; and K. Lyer for help with the initial phenotypic analysis. We also thank M. Tessier-Lavigne for the Slit1- and Slit2-knockout mice, J. Livet for the CAG:CreERTM mouse line and R. Adams for the Cdh5:CreERT2 mouse line. VEGF-A–AP constructs were provided by C. Ruiz de Almodovar (Vesalius Research Center, Leuven, Belgium). pGEX-PAK-CRIB was a kind gift from M. Schwartz (Yale University, New Haven, Connecticut, USA). The Slit2(lox) mutant mouse line was established at the Mouse Clinical Institute (Institut Clinique de la Souris, Strasbourg, France) in the Targeted Mutagenesis and Transgenesis Department.
Author information
Authors and Affiliations
Contributions
A.C. and A.E. supervised the project. A.C., A.E., N.R. and A.D. designed the experiments and wrote the manuscript. N.R., A.D., T.M., R.-A.N.C., G.G., B.C. and L.P.-F. performed the experiments and analyzed the data. L.M. generated the Robo1; Robo2lox mice.
Corresponding authors
Ethics declarations
Competing interests
This project was supported in part by a grant from Sanofi-Fovea to A.C.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13 and Supplementary Table 1 (PDF 2849 kb)
Rights and permissions
About this article
Cite this article
Rama, N., Dubrac, A., Mathivet, T. et al. Slit2 signaling through Robo1 and Robo2 is required for retinal neovascularization. Nat Med 21, 483–491 (2015). https://doi.org/10.1038/nm.3849
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3849
This article is cited by
-
Insights into myopic choroidal neovascularization based on quantitative proteomics analysis of the aqueous humor
BMC Genomics (2023)
-
An intronic variant in TBX4 in a single family with variable and severe pulmonary manifestations
npj Genomic Medicine (2023)
-
Genome-wide CRISPR knockout screening identified G protein pathway suppressor 2 as a novel tumor suppressor for uveal melanoma metastasis
Journal of Cancer Research and Clinical Oncology (2023)
-
Targeting Redundant ROBO1 and SDF-1 Pathways Prevents Adult Hemangioblast Derived-EPC and CEC Activity Effectively Blocking Tumor Neovascularization
Stem Cell Reviews and Reports (2023)
-
miR-152-3p impedes the malignant phenotypes of hepatocellular carcinoma by repressing roundabout guidance receptor 1
Cellular & Molecular Biology Letters (2022)