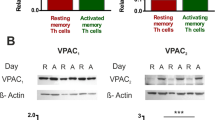
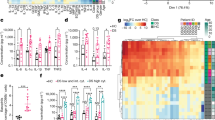
Abstract
During an inflammatory response, lymphocyte recruitment into tissue must be tightly controlled because dysregulated trafficking contributes to the pathogenesis of chronic disease. Here we show that during inflammation and in response to adiponectin, B cells tonically inhibit T cell trafficking by secreting a peptide (PEPITEM) proteolytically derived from 14.3.3 zeta delta (14.3.3.ζδ) protein. PEPITEM binds cadherin-15 on endothelial cells, promoting synthesis and release of sphingosine-1 phosphate, which inhibits trafficking of T cells without affecting recruitment of other leukocytes. Expression of adiponectin receptors on B cells and adiponectin-induced PEPITEM secretion wanes with age, implying immune senescence of the pathway. Additionally, these changes are evident in individuals with type 1 diabetes or rheumatoid arthritis, and circulating PEPITEM in patient serum is reduced compared to that of healthy age-matched donors. In both diseases, tonic inhibition of T cell trafficking across inflamed endothelium is lost. Control of patient T cell trafficking is re-established by treatment with exogenous PEPITEM. Moreover, in animal models of peritonitis, hepatic ischemia-reperfusion injury, Salmonella infection, uveitis and Sjögren's syndrome, PEPITEM reduced T cell recruitment into inflamed tissues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Cyster, J.G. Lymphoid organ development and cell migration. Immunol. Rev. 195, 5–14 (2003).
Koulmanda, M. et al. Modification of adverse inflammation is required to cure new-onset type 1 diabetic hosts. Proc. Natl. Acad. Sci. USA 104, 13074–13079 (2007).
Pober, J.S. & Cotran, R.S. The role of endothelial cells in inflammation. Transplantation 50, 537–544 (1990).
Kadowaki, T. et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest. 116, 1784–1792 (2006).
Ouedraogo, R. et al. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J. Clin. Invest. 117, 1718–1726 (2007).
Pang, T.T. et al. Inhibition of islet immunoreactivity by adiponectin is attenuated in human type 1 diabetes. J. Clin. Endocrinol. Metab. 98, E418–E428 (2013).
Pang, T.T. & Narendran, P. The distribution of adiponectin receptors on human peripheral blood mononuclear cells. Ann. NY Acad. Sci. 1150, 143–145 (2008).
Lalor, P.F., Shields, P., Grant, A. & Adams, D.H. Recruitment of lymphocytes to the human liver. Immunol. Cell Biol. 80, 52–64 (2002).
Olsson, R. & Carlsson, P.O. The pancreatic islet endothelial cell: emerging roles in islet function and disease. Int. J. Biochem. Cell Biol. 38, 710–714 (2006).
Andrew, D.P. et al. Transendothelial migration and trafficking of leukocytes in LFA-1-deficient mice. Eur. J. Immunol. 28, 1959–1969 (1998).
Brezinschek, R.I., Lipsky, P.E., Galea, P., Vita, R. & Oppenheimer-Marks, N. Phenotypic characterization of CD4+ T cells that exhibit a transendothelial migratory capacity. J. Immunol. 154, 3062–3077 (1995).
Cinamon, G., Shinder, V. & Alon, R. Shear forces promote lymphocyte migration across vascular endothelium bearing apical chemokines. Nat. Immunol. 2, 515–522 (2001).
Ley, K., Laudanna, C., Cybulsky, M.I. & Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 (2007).
Ma, L. et al. CD31 exhibits multiple roles in regulating T lymphocyte trafficking in vivo. J. Immunol. 189, 4104–4111 (2012).
Shulman, Z. et al. Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity 30, 384–396 (2009).
Springer, T.A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 57, 827–872 (1995).
McGettrick, H.M. et al. Direct observations of the kinetics of migrating T cells suggest active retention by endothelial cells with continual bidirectional migration. J. Leukoc. Biol. 85, 98–107 (2009).
Piali, L. et al. The chemokine receptor CXCR3 mediates rapid and shear-resistant adhesion-induction of effector T lymphocytes by the chemokines IP10 and Mig. Eur. J. Immunol. 28, 961–972 (1998).
Spiegel, S. & Milstien, S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 11, 403–415 (2011).
Jones, D.A., McIntire, L.V., Smith, C.W. & Picker, L.J. A two-step adhesion cascade for T cell/endothelial cell interactions under flow conditions. J. Clin. Invest. 94, 2443–2450 (1994).
Luscinskas, F.W., Ding, H. & Lichtman, A.H. P-selectin and vascular cell adhesion molecule 1 mediate rolling and arrest, respectively, of CD4+ T lymphocytes on tumor necrosis factor α–activated vascular endothelium under flow. J. Exp. Med. 181, 1179–1186 (1995).
Yago, T. et al. Analysis of an initial step of T cell adhesion to endothelial monolayers under flow conditions. J. Immunol. 154, 1216–1222 (1995).
Ahmed, S.R. et al. Prostaglandin D2 regulates CD4+ memory T cell trafficking across blood vascular endothelium and primes these cells for clearance across lymphatic endothelium. J. Immunol. 187, 1432–1439 (2011).
Fukuhara, S. et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J. Clin. Invest. 122, 1416–1426 (2012).
Hisano, Y., Kobayashi, N., Yamaguchi, A. & Nishi, T. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS ONE 7, e38941 (2012).
Nijnik, A. et al. The role of sphingosine-1-phosphate transporter Spns2 in immune system function. J. Immunol. 189, 102–111 (2012).
Ledgerwood, L.G. et al. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat. Immunol. 9, 42–53 (2008).
Mandala, S. et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296, 346–349 (2002).
Matloubian, M. et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004).
Pappu, R. et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 316, 295–298 (2007).
Pham, T.H., Okada, T., Matloubian, M., Lo, C.G. & Cyster, J.G. S1P1 receptor signaling overrides retention mediated by Gαi-coupled receptors to promote T cell egress. Immunity 28, 122–133 (2008).
Hammad, S.M., Al Gadban, M.A., Semler, A.J. & Klein, R.L. Sphingosine 1-phosphate distribution in human plasma: associations with lipid profiles. J. Lipids 2012, 180705 (2012).
Murata, N. et al. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem. J. 352, 809–815 (2000).
Sattler, K. & Levkau, B. Sphingosine-1-phosphate as a mediator of high-density lipoprotein effects in cardiovascular protection. Cardiovasc. Res. 82, 201–211 (2009).
Chen, J. et al. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int. Immunol. 5, 647–656 (1993).
Johansson, C. et al. Elevated neutrophil, macrophage and dendritic cell numbers characterize immune cell populations in mice chronically infected with Salmonella. Microb. Pathog. 41, 49–58 (2006).
Lalor, P.F., Lai, W.K., Curbishley, S.M., Shetty, S. & Adams, D.H. Human hepatic sinusoidal endothelial cells can be distinguished by expression of phenotypic markers related to their specialised functions in vivo. World J. Gastroenterol. 12, 5429–5439 (2006).
Wong, J. et al. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J. Clin. Invest. 99, 2782–2790 (1997).
Bombardieri, M. et al. Inducible tertiary lymphoid structures, autoimmunity, and exocrine dysfunction in a novel model of salivary gland inflammation in C57BL/6 mice. J. Immunol. 189, 3767–3776 (2012).
Bridges, D. & Moorhead, G.B. 14–3-3 proteins: a number of functions for a numbered protein. Sci. STKE 2005, re10 (2005).
Dougherty, M.K. & Morrison, D.K. Unlocking the code of 14–3-3. J. Cell Sci. 117, 1875–1884 (2004).
Fu, H., Subramanian, R.R. & Masters, S.C. 14–3-3 proteins: structure, function, and regulation. Annu. Rev. Pharmacol. Toxicol. 40, 617–647 (2000).
Layfield, R. et al. Neurofibrillary tangles of Alzheimer's disease brains contain 14-3-3 proteins. Neurosci. Lett. 209, 57–60 (1996).
Lodygin, D. & Hermeking, H. The role of epigenetic inactivation of 14-3-3σ in human cancer. Cell Res. 15, 237–246 (2005).
Garcia-Guzman, M., Dolfi, F., Russello, M. & Vuori, K. Cell adhesion regulates the interaction between the docking protein p130(Cas) and the 14-3-3 proteins. J. Biol. Chem. 274, 5762–5768 (1999).
Han, D.C., Rodriguez, L.G. & Guan, J.L. Identification of a novel interaction between integrin beta1 and 14-3-3β. Oncogene 20, 346–357 (2001).
Mhawech, P. 14-3-3 proteins–an update. Cell Res. 15, 228–236 (2005).
Rodriguez, L.G. & Guan, J.L. 14-3-3 regulation of cell spreading and migration requires a functional amphipathic groove. J. Cell. Physiol. 202, 285–294 (2005).
Choi, E.Y. et al. Regulation of LFA-1–dependent inflammatory cell recruitment by Cbl-b and 14-3-3 proteins. Blood 111, 3607–3614 (2008).
Fagerholm, S.C., Hilden, T.J., Nurmi, S.M. & Gahmberg, C.G. Specific integrin alpha and beta chain phosphorylations regulate LFA-1 activation through affinity-dependent and -independent mechanisms. J. Cell Biol. 171, 705–715 (2005).
Yuan, Y. et al. Identification of a novel 14-3-3ζ binding site within the cytoplasmic domain of platelet glycoprotein Ibα that plays a key role in regulating the von Willebrand factor binding function of glycoprotein Ib-IX. Circ. Res. 105, 1177–1185 (2009).
Roviezzo, F. et al. Sphingosine-1-phosphate modulates vascular permeability and cell recruitment in acute inflammation in vivo. J. Pharmacol. Exp. Ther. 337, 830–837 (2011).
Mehta, D., Konstantoulaki, M., Ahmmed, G.U. & Malik, A.B. Sphingosine 1-phosphate-induced mobilization of intracellular Ca2+ mediates Rac activation and adherens junction assembly in endothelial cells. J. Biol. Chem. 280, 17320–17328 (2005).
LeBien, T.W. & Tedder, T.F. B lymphocytes: how they develop and function. Blood 112, 1570–1580 (2008).
Serreze, D.V. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new 'speed congenic' stock of NOD.Ig mu null mice. J. Exp. Med. 184, 2049–2053 (1996).
Finnegan, A., Ashaye, S. & Hamel, K.M. B effector cells in rheumatoid arthritis and experimental arthritis. Autoimmunity 45, 353–363 (2012).
Pescovitz, M.D. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N. Engl. J. Med. 361, 2143–2152 (2009).
De Vita, S. et al. Efficacy of selective B cell blockade in the treatment of rheumatoid arthritis. Evidence of a pathogenic role for B cells. Arthritis Rheum. 46, 2029–2033 (2002).
Rainger, G.E., Stone, P., Morland, C.M. & Nash, G.B. A novel system for investigating the ability of smooth muscle cells and fibroblasts to regulate adhesion of flowing leukocytes to endothelial cells. J. Immunol. Methods 255, 73–82 (2001).
Cooke, B.M., Usami, S., Perry, I. & Nash, G.B. A simplified method for culture of endothelial cells and analysis of adhesion of blood cells under conditions of flow. Microvasc. Res. 45, 33–45 (1993).
Butler, L.M., Rainger, G.E., Rahman, M. & Nash, G.B. Prolonged culture of endothelial cells and deposition of basement membrane modify the recruitment of neutrophils. Exp. Cell Res. 310, 22–32 (2005).
Eidhammer, I. et al. Computational and Statistical Methods for Protein Quantification by Mass Spectrometry (John Wiley & Sons, 2012).
Rajakariar, R. et al. Novel biphasic role for lymphocytes revealed during resolving inflammation. Blood 111, 4184–4192 (2008).
Hoiseth, S.K. & Stocker, B.A. Aromatic-dependent Salmonella Typhimurium are non-virulent and effective as live vaccines. Nature 291, 238–239 (1981).
Cunningham, A.F. et al. Responses to the soluble flagellar protein FliC are Th2, while those to FliC on Salmonella are Th1. Eur. J. Immunol. 34, 2986–2995 (2004).
Cascalho, M. et al. A quasi-monoclonal mouse. Science 272, 1649–1652 (1996).
Flores-Langarica, A. et al. Systemic flagellin immunization stimulates mucosal CD103+ dendritic cells and drives Foxp3+ regulatory T cell and IgA responses in the mesenteric lymph node. J. Immunol. 189, 5745–5754 (2012).
Kerr, E.C. et al. Analysis of retinal cellular infiltrate in experimental autoimmune uveoretinitis reveals multiple regulatory cell populations. J. Autoimmun. 31, 354–361 (2008).
Alberti, K.G. & Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 15, 539–553 (1998).
Acknowledgements
The authors thank A. Sams and S. Tullin from Novo Nordisk for providing high-molecular-weight recombinant adiponectin. We are grateful to N. Shimwell and D. Ward for assistance with mass spectrometry, and to G. Pui Choy for her help with animal experiments (all from the University of Birmingham, UK). Thanks also to N. Hogg (Cancer Research UK, London Research Institute, UK) for the activation epitope–sensitive anti-LFA-1 antibody, KIM127. We also thank C. McDonnel (GE Healthcare) for her help with the Biacore experiments and R.A. Kingsley for the Salmonella Typhimurium strain SL3261 (Wellcome Trust Sanger Institute, Cambridge, UK). We also thank D. Hardie for helping with the automated cell sorting (University of Birmingham, UK) and the University of Bristol Flow Cytometry Facility. We thank P. Nightingale (University Hospitals Birmingham NHS Trust) for advice on statistical analysis. This work was supported by grants from Diabetes UK (P.N., G.E.R.) (097825/Z/11/A), the Wellcome Trust (P.N., G.E.R.; ISSF 12/13-097825/Z/11/A), an Early Career Award from the Society for Endocrinology (M.C.), the Medical Research Council (CIC 12011) a senior fellowship for L.S.K.W. (G0802382) and the Juvenile Diabetes Research Foundation (P.N., G.E.R.; 5-2013-207). Work in the laboratories of G.E.R. is supported by the British Heart Foundation at Project grant (PG/11/49/28983) and Programme grant (RG/12/7/29693) level. H.M.M. was supported by an Arthritis Research UK Career Development Fellowship (19899). A.K. was supported by a National Institute for Health Research, Research for Patient Benefit (PB-PG-0609-19093). F.B. is supported by a Wellcome Trust Clinician Scientist Fellowship. A.F. was supported by an Arthritis Research UK Clinician Scientist Fellowship (18547). The research leading to the rheumatoid arthritis subject data was funded within the FP7 HEALTH programme under the grant agreement FP7-HEALTH-F2-2012-305549. This report is independent research which was partly supported by the National Institute for Health Research/Wellcome Trust Clinical Research Facility at University Hospitals Birmingham NHS Foundation Trust. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Author information
Authors and Affiliations
Contributions
M.C. conceived and performed experiments, analyzed and interpreted the data, and co-wrote the manuscript. H.M.M. conceived, performed experiments, analyzed and interpreted the data. B.A., S.J.K., C.M.Y., A.K., A.O., M.A., M.H., S.N., J.R.H., D.A.C. and J.R. performed experiments and analyzed the data. A.M., F.B., A.F.C., K.R., A.F., D.A.C., A.D.D., N.K., L.S.K.W., C.D.B. and G.B.N. organized and conducted the study, including analysis, data interpretation and critique of the manuscript. K.R. and A.F. recruited and diagnosed patients in early arthritis clinics, and acquired the clinical data. P.N. conceived, designed and organized the T1D study and analyzed and interpreted the data. G.E.R. conceived, designed, organized and conducted the study, including analysis and interpretation of data, and co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
M.C., H.M.M., P.N. and G.E.R. hold patents for the therapeutic and diagnostic use of PEPITEM (WO2013/104928) and cadherin-15 (WO2015/001356) in autoimmune disease, chronic inflammatory disease and other diseases in which T cells contribute to pathogenesis, as well as for the use of adipoRs expression as a biomarker for rheumatoid arthritis (WO2014/080204). The other authors have no competing financial interests to declare.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Tables 1–8. (PDF 855 kb)
Rights and permissions
About this article
Cite this article
Chimen, M., McGettrick, H., Apta, B. et al. Homeostatic regulation of T cell trafficking by a B cell–derived peptide is impaired in autoimmune and chronic inflammatory disease. Nat Med 21, 467–475 (2015). https://doi.org/10.1038/nm.3842
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3842
This article is cited by
-
Subtractive panning for the isolation of monoclonal PEPITEM peptide antibody by phage display
Scientific Reports (2023)
-
The Sphingosine 1-Phosphate Axis: an Emerging Therapeutic Opportunity for Endometriosis
Reproductive Sciences (2023)
-
The Role of B Cells in Cardiomyopathy and Heart Failure
Current Cardiology Reports (2022)
-
Targeting regulatory T cells for immunotherapy in melanoma
Molecular Biomedicine (2021)
-
Plasma apolipopotein C-2 elevation is associated with Takayasu arteritis
Scientific Reports (2021)