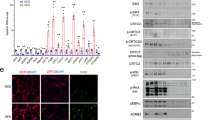
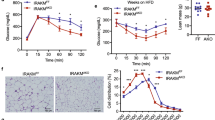
Abstract
Cigarette smoking promotes body weight reduction in humans while paradoxically also promoting insulin resistance (IR) and hyperinsulinemia. However, the mechanisms behind these effects are unclear. Here we show that nicotine, a major constituent of cigarette smoke, selectively activates AMP-activated protein kinase α2 (AMPKα2) in adipocytes, which in turn phosphorylates MAP kinase phosphatase-1 (MKP1) at serine 334, initiating its proteasome-dependent degradation. The nicotine-dependent reduction of MKP1 induces the aberrant activation of both p38 mitogen–activated protein kinase and c-Jun N-terminal kinase, leading to increased phosphorylation of insulin receptor substrate 1 (IRS1) at serine 307. Phosphorylation of IRS1 leads to its degradation, protein kinase B inhibition, and the loss of insulin-mediated inhibition of lipolysis. Consequently, nicotine increases lipolysis, which results in body weight reduction, but this increase also elevates the levels of circulating free fatty acids and thus causes IR in insulin-sensitive tissues. These results establish AMPKα2 as an essential mediator of nicotine-induced whole-body IR in spite of reductions in adiposity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Dubé, J.J. et al. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia 54, 1147–1156 (2011).
Iribarren, C., Tekawa, I.S., Sidney, S. & Friedman, G.D. Effect of cigar smoking on the risk of cardiovascular disease, chronic obstructive pulmonary disease, and cancer in men. N. Engl. J. Med. 340, 1773–1780 (1999).
Eliasson, B., Taskinen, M.R. & Smith, U. Long-term use of nicotine gum is associated with hyperinsulinemia and insulin resistance. Circulation 94, 878–881 (1996).
Wack, J.T. & Rodin, J. Smoking and its effects on body weight and the systems of caloric regulation. Am. J. Clin. Nutr. 35, 366–380 (1982).
Flegal, K.M., Troiano, R.P., Pamuk, E.R., Kuczmarski, R.J. & Campbell, S.M. The influence of smoking cessation on the prevalence of overweight in the United-States. N. Engl. J. Med. 333, 1165–1170 (1995).
Williamson, D.F. et al. Smoking cessation and severity of weight-gain in a national cohort. N. Engl. J. Med. 324, 739–745 (1991).
Schnoll, R.A., Goren, A., Annunziata, K. & Suaya, J.A. The prevalence, predictors and associated health outcomes of high nicotine dependence using three measures among US smokers. Addiction 108, 1989–2000 (2013).
Johnson, G.L. & Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298, 1911–1912 (2002).
Rotter, V., Nagaev, I. & Smith, U. Interleukin-6 (IL-6) induces insulin resistance in 3T3–L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J. Biol. Chem. 278, 45777–45784 (2003).
Hiratani, K. et al. Roles of mTOR and JNK in serine phosphorylation, translocation, and degradation of IRS-1. Biochem. Biophys. Res. Commun. 335, 836–842 (2005).
Hirosumi, J. et al. A central role for JNK in obesity and insulin resistance. Nature 420, 333–336 (2002).
Bennett, B.L., Satoh, Y. & Lewis, A.J. JNK: a new therapeutic target for diabetes. Curr. Opin. Pharmacol. 3, 420–425 (2003).
Fernández-Galilea, M., Perez-Matute, P., Prieto-Hontoria, P.L., Martinez, J.A. & Moreno-Aliaga, M.J. Effects of lipoic acid on lipolysis in 3T3–L1 adipocytes. J. Lipid Res. 53, 2296–2306 (2012).
Heusch, W.L. & Maneckjee, R. Signalling pathways involved in nicotine regulation of apoptosis of human lung cancer cells. Carcinogenesis 19, 551–556 (1998).
Nakamura, S. et al. Nicotine induces upregulated expression of beta defensin-2 via the p38MAPK pathway in the HaCaT human keratinocyte cell line. Med. Mol. Morphol. 43, 204–210 (2010).
Li, J.M. et al. Nicotine enhances angiotensin II-induced mitogenic response in vascular smooth muscle cells and fibroblasts. Arterioscler. Thromb. Vasc. Biol. 24, 80–84 (2004).
Salminen, A., Hyttinen, J.M. & Kaarniranta, K. AMP-activated protein kinase inhibits NF-kappaB signaling and inflammation: impact on healthspan and lifespan. J. Mol. Med. 89, 667–676 (2011).
Steinberg, G.R. & Kemp, B.E. AMPK in health and disease. Physiol. Rev. 89, 1025–1078 (2009).
An, Z.B. et al. Nicotine-induced activation of AMP-activated protein kinase inhibits fatty acid synthase in 3T3L1 adipocytes—a role for oxidant stress. J. Biol. Chem. 282, 26793–26801 (2007).
Wang, S. et al. Activation of AMP-activated protein kinase alpha2 by nicotine instigates formation of abdominal aortic aneurysms in mice in vivo. Nat. Med. 18, 902–910 (2012).
Martínez de Morentin, P.B. et al. Nicotine induces negative energy balance through hypothalamic AMP-activated protein kinase. Diabetes 61, 807–817 (2012).
Benowitz, N.L. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Prog. Cardiovasc. Dis. 46, 91–111 (2003).
Tundulawessa, Y., Yongchaiyud, P., Chutrthong, W. & Tundulawessa, K. The bioequivalent and effect of nicotine formulation gum on smoking cessation. J. Med. Assoc. Thailand (Chotmaihet thangphaet) 93, 574–579 (2010).
Jocken, J.W. et al. Insulin-mediated suppression of lipolysis in adipose tissue and skeletal muscle of obese type 2 diabetic men and men with normal glucose tolerance. Diabetologia 56, 2255–2265 (2013).
Mineur, Y.S. et al. Nicotine decreases food intake through activation of POMC neurons. Science 332, 1330–1332 (2011).
Pal, D. et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat. Med. 18, 1279–1285 (2012).
Liew, C.W. et al. Ablation of TRIP-Br2, a regulator of fat lipolysis, thermogenesis and oxidative metabolism, prevents diet-induced obesity and insulin resistance. Nat. Med. 19, 217–226 (2013).
Guo, W. et al. Acipimox, an inhibitor of lipolysis, attenuates atherogenesis in LDLR-null mice treated with HIV protease inhibitor ritonavir. Arterioscler. Thromb. Vasc. Biol. 29, 2028–2032 (2009).
Hardie, D.G., Ross, F.A. & Hawley, S.A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 13, 251–262 (2012).
Fullerton, M.D. et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 19, 1649–1654 (2013).
Ruderman, N.B., Carling, D., Prentki, M. & Cacicedo, J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Invest. 123, 2764–2772 (2013).
Musi, N. et al. AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise. Diabetes 50, 921–927 (2001).
Garton, A.J. et al. Phosphorylation of bovine hormone-sensitive lipase by the AMP-activated protein kinase. A possible antilipolytic mechanism. Eur. J Biochemistry 179, 249–254 (1989).
Daval, M. et al. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J. Biol. Chem. 280, 25250–25257 (2005).
Bourron, O. et al. Biguanides and thiazolidinediones inhibit stimulated lipolysis in human adipocytes through activation of AMP-activated protein kinase. Diabetologia 53, 768–778 (2010).
Djouder, N. et al. PKA phosphorylates and inactivates AMPKalpha to promote efficient lipolysis. EMBO J. 29, 469–481 (2010).
Ahmadian, M. et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 13, 739–748 (2011).
Lin, L. et al. Adipocyte expression of PU.1 transcription factor causes insulin resistance through upregulation of inflammatory cytokine gene expression and ROS production. Am. J. Physiol. Endocrinol. Metab. 302, E1550–E1559 (2012).
Um, S.H. et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431, 200–205 (2004).
Horike, N. et al. Adipose-specific expression, phosphorylation of Ser794 in insulin receptor substrate-1, and activation in diabetic animals of salt-inducible kinase-2. J. Biol. Chem. 278, 18440–18447 (2003).
Sánchez-Tillo, E. et al. JNK1 Is required for the induction of Mkp1 expression in macrophages during proliferation and lipopolysaccharide-dependent activation. J. Biol. Chem. 282, 12566–12573 (2007).
Brondello, J.M., Pouyssegur, J. & McKenzie, F.R. Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science 286, 2514–2517 (1999).
Wu, J.J. et al. Mice lacking MAP kinase phosphatase-1 have enhanced MAP kinase activity and resistance to diet-induced obesity. Cell Metab. 4, 61–73 (2006).
Zhong, C., Talmage, D.A. & Role, L.W. Nicotine elicits prolonged calcium signaling along ventral hippocampal axons. PLoS ONE 8, e82719 (2013).
Hogg, R.C. & Bertrand, D. Neuroscience. What genes tell us about nicotine addiction. Science 306, 983–985 (2004).
Friedman, T.C. et al. Additive effects of nicotine and high-fat diet on hepatic steatosis in male mice. Endocrinology 153, 5809–5820 (2012).
Seoane-Collazo, P. et al. Nicotine improves obesity and hepatic steatosis and ER stress in diet-induced obese male rats. Endocrinology 155, 1679–1689 (2014).
Xu, T.Y. et al. Chronic exposure to nicotine enhances insulin sensitivity through alpha 7 nicotinic acetylcholine receptor-STAT3 pathway. PLoS ONE 7, e51217 (2012).
Yoon, M.J. et al. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes 55, 2562–2570 (2006).
Lin, Y.W. & Yang, J.L. Cooperation of ERK and SCFSkp2 for MKP-1 destruction provides a positive feedback regulation of proliferating signaling. J. Biol. Chem. 281, 915–926 (2006).
Choi, S.M. et al. Insulin regulates adipocyte lipolysis via an Akt-independent signaling pathway. Mol. Cell. Biol. 30, 5009–5020 (2010).
Villena, J.A. et al. Induced adiposity and adipocyte hypertrophy in mice lacking the AMP-activated protein kinase-alpha2 subunit. Diabetes 53, 2242–2249 (2004).
Bolinder, J., Sjoberg, S. & Arner, P. Stimulation of adipose tissue lipolysis following insulin-induced hypoglycaemia: evidence of increased beta-adrenoceptor-mediated lipolytic response in IDDM. Diabetologia 39, 845–853 (1996).
Wolffenbuttel, B.H., Weber, R.F., van Koetsveld, P.M., Weeks, L. & Verschoor, L. A randomized crossover study of sulphonylurea and insulin treatment in patients with type 2 diabetes poorly controlled on dietary therapy. Diabet. Med. 6, 520–525 (1989).
Gabrielsson, J. & Bondesson, U. Constant-rate infusion of nicotine and cotinine. I. A physiological pharmacokinetic analysis of the cotinine disposition, and effects on clearance and distribution in the rat. J. Pharmacokinet. Biopharm. 15, 583–599 (1987).
Song, P. et al. Adenosine monophosphate-activated protein kinase-alpha2 deficiency promotes vascular smooth muscle cell migration via S-phase kinase-associated protein 2 upregulation and E-cadherin downregulation. Arterioscler. Thromb. Vasc. Biol. 33, 2800–2809 (2013).
Dorfman, K. et al. Disruption of the erp/mkp-1 gene does not affect mouse development: normal MAP kinase activity in ERP/MKP-1-deficient fibroblasts. Oncogene 13, 925–931 (1996).
Blättler, S.M. et al. Yin Yang 1 deficiency in skeletal muscle protects against rapamycin-induced diabetic-like symptoms through activation of insulin/IGF signaling. Cell Metab. 15, 505–517 (2012).
Turner, N. et al. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 56, 1638–1648 (2013).
Li, P. et al. Adipocyte NCoR knockout decreases PPARgamma phosphorylation and enhances PPARgamma activity and insulin sensitivity. Cell 147, 815–826 (2011).
Zhang, W., Wang, Q., Song, P. & Zou, M.H. Liver kinase b1 is required for white adipose tissue growth and differentiation. Diabetes 62, 2347–2358 (2013).
Targher, G. et al. Cigarette smoking and insulin resistance in patients with noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 82, 3619–3624 (1997).
Coburn, C.T. et al. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J. Biol. Chem. 275, 32523–32529 (2000).
Siri, P. et al. Post-transcriptional stimulation of the assembly and secretion of triglyceride-rich apolipoprotein B lipoproteins in a mouse with selective deficiency of brown adipose tissue, obesity, and insulin resistance. J. Biol. Chem. 276, 46064–46072 (2001).
Wang, S., Song, P. & Zou, M.H. Inhibition of AMP-activated protein kinase alpha (AMPKα) by doxorubicin accentuates genotoxic stress and cell death in mouse embryonic fibroblasts and cardiomyocytes: role of p53 and SIRT1. J. Biol. Chem. 287, 8001–8012 (2012).
Xie, Z.L. et al. Identification of the serine 307 of LKB1 as a novel phosphorylation site essential for its nucleocytoplasmic transport and endothelial cell angiogenesis. Mol. Cell. Biol. 29, 3582–3596 (2009).
Acknowledgements
We thank L. Yu for helpful discussions and D. Wang and Y. Du for technical support. Prkaa1flox/flox and Prkaa2flox/flox mice were provided by B. Viollet (INSERM, U1016, Institut Cochin, Paris, France). This study was supported by grants from the US National Institutes of Health (HL079584, HL080499, HL074399, HL089920, HL096032, HL105157, HL110488, and AG047776 to M.-H.Z.) and (HL128014 to Z.X.). This study was also supported in part by grants from the National Natural Science Foundation of China (81100209, 81025002 and 91339116 to Z.Y. and 81270355 to J.W.), the Scientist Development Grant of American Heart Association (11SDG5560036 to P.S.), and the Oklahoma Center for the Advancement of Science and Technology (HR12-061 to P.S.).
Author information
Authors and Affiliations
Contributions
Y.W. designed and performed the experiments, analyzed data, and drafted the manuscript. P.S., W.Z., X.D., Z.L., C.O., Z.X. and X.Z. performed a part of the animal experiments. J.L. and Z.Y. performed the human experiments. Z.Z. and J.W. partially performed the in vitro experiments, W.Z. and Q.L. generated series mutants. B.V. and M.F. provided the Ampk knockout mice. M.-H.Z. conceived the projects, designed the experiments, analyzed data, and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary figures and text
Supplementary Figures 1–8 and Supplementary Tables 1–3 (PDF 1663 kb)
Rights and permissions
About this article
Cite this article
Wu, Y., Song, P., Zhang, W. et al. Activation of AMPKα2 in adipocytes is essential for nicotine-induced insulin resistance in vivo. Nat Med 21, 373–382 (2015). https://doi.org/10.1038/nm.3826
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3826
This article is cited by
-
Gut microbial metabolites reveal diet-dependent metabolic changes induced by nicotine administration
Scientific Reports (2024)
-
The controversial effect of smoking and nicotine in SARS-CoV-2 infection
Allergy, Asthma & Clinical Immunology (2023)
-
The lipolysis inhibitor acipimox reverses the cardiac phenotype induced by electronic cigarettes
Scientific Reports (2023)
-
MKP1 promotes nonalcoholic steatohepatitis by suppressing AMPK activity through LKB1 nuclear retention
Nature Communications (2023)
-
Chromogranin A and its derived peptides: potential regulators of cholesterol homeostasis
Cellular and Molecular Life Sciences (2023)