Abstract
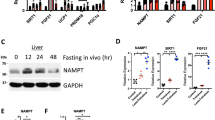
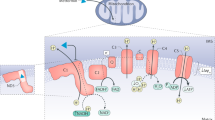
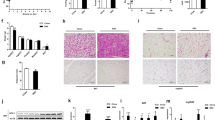
Resveratrol improves insulin sensitivity and lowers hepatic glucose production (HGP) in rat models of obesity and diabetes1,2,3,4,5, but the underlying mechanisms for these antidiabetic effects remain elusive. One process that is considered a key feature of resveratrol action is the activation of the nicotinamide adenine dinucleotide (NAD+)–dependent deacetylase sirtuin 1 (SIRT1) in various tissues1,3,6,7,8. However, the low bioavailability of resveratrol raises questions about whether the antidiabetic effects of oral resveratrol can act directly on these tissues9,10. We show here that acute intraduodenal infusion of resveratrol reversed a 3 d high fat diet (HFD)–induced reduction in duodenal–mucosal Sirt1 protein levels while also enhancing insulin sensitivity and lowering HGP. Further, we found that duodenum-specific knockdown of Sirt1 expression for 14 d was sufficient to induce hepatic insulin resistance in rats fed normal chow. We also found that the glucoregulatory role of duodenally acting resveratrol required activation of Sirt1 and AMP-activated protein kinase (Ampk) in this tissue to initiate a gut–brain–liver neuronal axis that improved hypothalamic insulin sensitivity and in turn, reduced HGP. In addition to the effects of duodenally acting resveratrol in an acute 3 d HFD–fed model of insulin resistance, we also found that short-term infusion of resveratrol into the duodenum lowered HGP in two other rat models of insulin resistance—a 28 d HFD–induced model of obesity and a nicotinamide (NA)–streptozotocin (STZ)–HFD-induced model of mild type 2 diabetes. Together, these studies highlight the therapeutic relevance of targeting duodenal SIRT1 to reverse insulin resistance and improve glucose homeostasis in obesity and diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Baur, J.A. et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444, 337–342 (2006).
Knight, C.M. et al. Mediobasal hypothalamic SIRT1 is essential for resveratrol's effects on insulin action in rats. Diabetes 60, 2691–2700 (2011).
Lagouge, M. et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127, 1109–1122 (2006).
Ramadori, G. et al. Central administration of resveratrol improves diet-induced diabetes. Endocrinology 150, 5326–5333 (2009).
Rivera, L., Moron, R., Zarzuelo, A. & Galisteo, M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem. Pharmacol. 77, 1053–1063 (2009).
Timmers, S. et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 14, 612–622 (2011).
Price, N.L. et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 15, 675–690 (2012).
Park, S.J. et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 148, 421–433 (2012).
Liang, L. et al. Pharmacokinetics, tissue distribution and excretion study of resveratrol and its prodrug 3,5,4′-tri-O-acetylresveratrol in rats. Phytomedicine 20, 558–563 (2013).
Sale, S. et al. Pharmacokinetics in mice and growth-inhibitory properties of the putative cancer chemopreventive agent resveratrol and the synthetic analogue trans 3,4,5,4′-tetramethoxystilbene. Br. J. Cancer 90, 736–744 (2004).
Sun, C. et al. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 6, 307–319 (2007).
Bhatt, J.K., Thomas, S. & Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 32, 537–541 (2012).
Brasnyó, P. et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 106, 383–389 (2011).
Crandall, J.P. et al. Pilot study of resveratrol in older adults with impaired glucose tolerance. J. Gerontol. A Biol. Sci. Med. Sci. 67, 1307–1312 (2012).
Banks, A.S. et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 8, 333–341 (2008).
Bordone, L. et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 6, 759–767 (2007).
Pfluger, P.T., Herranz, D., Velasco-Miguel, S., Serrano, M. & Tschop, M.H. Sirt1 protects against high-fat diet-induced metabolic damage. Proc. Natl. Acad. Sci. USA 105, 9793–9798 (2008).
Ramadori, G. et al. SIRT1 deacetylase in SF1 neurons protects against metabolic imbalance. Cell Metab. 14, 301–312 (2011).
Hubbard, B.P. et al. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 339, 1216–1219 (2013).
Patel, K.R. et al. Sulfate metabolites provide an intracellular pool for resveratrol generation and induce autophagy with senescence. Sci. Transl. Med. 5, 205ra133 (2013).
Firestein, R. et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS ONE 3, e2020 (2008).
Breen, D.M. et al. Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat. Med. 18, 950–955 (2012).
Côté, C.D., Zadeh-Tahmasebi, M., Rasmussen, B.A., Duca, F.A. & Lam, T.K. Hormonal signaling in the gut. J. Biol. Chem. 289, 11642–11649 (2014).
Rasmussen, B.A. et al. Jejunal leptin-PI3K signaling lowers glucose production. Cell Metab. 19, 155–161 (2014).
Wang, P.Y. et al. Upper intestinal lipids trigger a gut–brain–liver axis to regulate glucose production. Nature 452, 1012–1016 (2008).
Schwartz, M.W. et al. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature 503, 59–66 (2013).
Rodgers, J.T. et al. Nutrient control of glucose homeostasis through a complex of PGC-1 alpha and SIRT1. Nature 434, 113–118 (2005).
Wang, J. et al. Overfeeding rapidly induces leptin and insulin resistance. Diabetes 50, 2786–2791 (2001).
Baur, J.A. & Sinclair, D.A. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 5, 493–506 (2006).
Um, J.H. et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 59, 554–563 (2010).
Duca, F.A. et al. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat. Med. (2015) (in the press).
Rasmussen, B.A. et al. Duodenal activation of cAMP-dependent protein kinase induces vagal afferent firing and lowers glucose production in rats. Gastroenterology 142, 834–843.e3 (2012).
Chen, P.C., Kryukova, Y.N. & Shyng, S.L. Leptin regulates KATP channel trafficking in pancreatic beta-cells by a signaling mechanism involving AMP-activated protein kinase (AMPK) and cAMP-dependent protein kinase (PKA). J. Biol. Chem. 288, 34098–34109 (2013).
Pocai, A. et al. Hypothalamic K(ATP) channels control hepatic glucose production. Nature 434, 1026–1031 (2005).
Samuel, V.T. et al. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with Type 2 Diabetes. Proc. Natl. Acad. Sci. USA 106, 12121–12126 (2009).
Poulsen, M.M. et al. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes 62, 1186–1195 (2013).
Yoshino, J. et al. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 16, 658–664 (2012).
Cantó, C. et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458, 1056–1060 (2009).
Fulco, M. et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev. Cell 14, 661–673 (2008).
Ono, H. et al. Activation of hypothalamic S6 kinase mediates diet-induced hepatic insulin resistance in rats. J. Clin. Invest. 118, 2959–2968 (2008).
Kokorovic, A. et al. Duodenal mucosal protein kinase C-δ regulates glucose production in rats. Gastroenterology 141, 1720–1727 (2011).
Filippi, B.M., Yang, C.S., Tang, C. & Lam, T.K. Insulin activates Erk1/2 signaling in the dorsal vagal complex to inhibit glucose production. Cell Metab. 16, 500–510 (2012).
Sakamoto, K. et al. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 24, 1810–1820 (2005).
Dale, S., Wilson, W.A., Edelman, A.M. & Hardie, D.G. Similar substrate recognition motifs for mammalian Amp-activated protein kinase, higher-plant HMG-CoA reductase kinase-A, yeast SNF1, and mammalian calmodulin-dependent protein kinase I. FEBS Lett. 361, 191–195 (1995).
Acknowledgements
The authors are grateful to E. Burdett for technical assistance and to L. Schäffer (Novo Nordisk) for providing us with the insulin receptor antagonist (S961). This work is supported by a research grant to T.K.T.L. from the Canadian Institute of Health Research (MOP-82701). C.D.C. is supported by a Banting and Best Diabetes Centre graduate studentship. B.A.R. is supported by a Canadian Institute of Health Research Doctoral Vanier Canada scholarship. F.A.D. is a Banting Fellow. M.Z.-T. is supported by a Banting and Best Diabetes Centre graduate studentship. D.M.B. is supported by a Canadian Diabetes Association postdoctoral fellowship. T.K.T.L. holds the John Kitson McIvor (1915–1942) Endowed Chair in Diabetes Research and the Canada Research Chair in Obesity at the Toronto General Research Institute and the University of Toronto.
Author information
Authors and Affiliations
Contributions
C.D.C., B.A.R. and F.A.D. conducted and designed experiments, performed data analyses and wrote the manuscript; M.Z.-T., M.D., D.M.B. and B.M.F. assisted with experiments; J.A.B. assisted with the in vitro Sirt1 activity experiments; and T.K.T.L. supervised the project, designed experiments and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–3 and Supplementary Figures 1–5 (PDF 2337 kb)
Rights and permissions
About this article
Cite this article
Côté, C., Rasmussen, B., Duca, F. et al. Resveratrol activates duodenal Sirt1 to reverse insulin resistance in rats through a neuronal network. Nat Med 21, 498–505 (2015). https://doi.org/10.1038/nm.3821
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3821
This article is cited by
-
Small intestinal CaSR-dependent and CaSR-independent protein sensing regulates feeding and glucose tolerance in rats
Nature Metabolism (2024)
-
Resveratrol intervention attenuates chylomicron secretion via repressing intestinal FXR-induced expression of scavenger receptor SR-B1
Nature Communications (2023)
-
Resveratrol rescues cutaneous radiation-induced DNA damage via a novel AMPK/SIRT7/HMGB1 regulatory axis
Cell Death & Disease (2023)
-
A gut feeling for drugs that have metabolic benefits
Nature Communications (2023)
-
Hyperglycemia is associated with duodenal dysbiosis and altered duodenal microenvironment
Scientific Reports (2023)