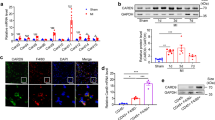
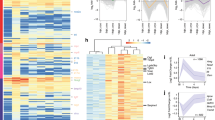
Abstract
Cardiac healing after myocardial ischemia depends on the recruitment and local expansion of myeloid cells, particularly macrophages. Here we identify Reg3β as an essential regulator of macrophage trafficking to the damaged heart. Using mass spectrometry–based secretome analysis, we found that dedifferentiating cardiomyocytes release Reg3β in response to the cytokine OSM, which signals through Jak1 and Stat3. Loss of Reg3β led to a large decrease in the number of macrophages in the ischemic heart, accompanied by increased ventricular dilatation and insufficient removal of neutrophils. This defect in neutrophil removal in turn caused enhanced matrix degradation, delayed collagen deposition and increased susceptibility to cardiac rupture. Our data indicate that OSM, acting through distinct intracellular pathways, regulates both cardiomyocyte dedifferentiation and cardiomyocyte-dependent regulation of macrophage trafficking. Release of OSM from infiltrating neutrophils and macrophages initiates a positive feedback loop in which OSM-induced production of Reg3β in cardiomyocytes attracts additional OSM-secreting macrophages. The activity of the feedback loop controls the degree of macrophage accumulation in the heart, which is instrumental in myocardial healing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Frangogiannis, N.G., Smith, C.W. & Entman, M.L. The inflammatory response in myocardial infarction. Cardiovasc. Res. 53, 31–47 (2002).
Frangogiannis, N.G. Regulation of the inflammatory response in cardiac repair. Circ. Res. 110, 159–173 (2012).
Lambert, J.M., Lopez, E.F. & Lindsey, M.L. Macrophage roles following myocardial infarction. Int. J. Cardiol. 130, 147–158 (2008).
Biasucci, L.M. et al. Elevated levels of interleukin-6 in unstable angina. Circulation 94, 874–877 (1996).
Fisman, E.Z. et al. Interleukin-6 and the risk of future cardiovascular events in patients with angina pectoris and/or healed myocardial infarction. Am. J. Cardiol. 98, 14–18 (2006).
Frangogiannis, N.G. The immune system and cardiac repair. Pharmacol. Res. 58, 88–111 (2008).
Nahrendorf, M. et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 (2007).
Dobaczewski, M., Gonzalez-Quesada, C. & Frangogiannis, N.G. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J. Mol. Cell. Cardiol. 48, 504–511 (2010).
Epelman, S. et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40, 91–104 (2014).
van Amerongen, M.J., Harmsen, M.C., van Rooijen, N., Petersen, A.H. & van Luyn, M.J. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am. J. Pathol. 170, 818–829 (2007).
Irwin, M.W. et al. Tissue expression and immunolocalization of tumor necrosis factor-alpha in postinfarction dysfunctional myocardium. Circulation 99, 1492–1498 (1999).
Iversen, P.O., Nicolaysen, G. & Sioud, M. DNA enzyme targeting TNF-alpha mRNA improves hemodynamic performance in rats with postinfarction heart failure. Am. J. Physiol. Heart Circ. Physiol. 281, H2211–H2217 (2001).
Leuschner, F. et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J. Exp. Med. 209, 123–137 (2012).
Baggiolini, M. Chemokines and leukocyte traffic. Nature 392, 565–568 (1998).
Frangogiannis, N.G. Chemokines in the ischemic myocardium: from inflammation to fibrosis. Inflamm. Res. 53, 585–595 (2004).
Frangogiannis, N.G. The role of the chemokines in myocardial ischemia and reperfusion. Curr. Vasc. Pharmacol. 2, 163–174 (2004).
Dobaczewski, M. & Frangogiannis, N.G. Chemokines in myocardial infarction: translating basic research into clinical medicine. Future Cardiol. 4, 347–351 (2008).
Grenier, A. et al. Oncostatin M production and regulation by human polymorphonuclear neutrophils. Blood 93, 1413–1421 (1999).
Radka, S.F., Nakamura, S., Sakurada, S. & Salahuddin, S.Z. Correlation of oncostatin M secretion by human retrovirus-infected cells with potent growth stimulation of cultured spindle cells from AIDS-Kaposi's sarcoma. J. Immunol. 150, 5195–5201 (1993).
Brown, T.J., Lioubin, M.N. & Marquardt, H. Purification and characterization of cytostatic lymphokines produced by activated human T lymphocytes. Synergistic antiproliferative activity of transforming growth factor beta 1, interferon-gamma, and oncostatin M for human melanoma cells. J. Immunol. 139, 2977–2983 (1987).
Kubin, T. et al. Oncostatin M is a major mediator of cardiomyocyte dedifferentiation and remodeling. Cell Stem Cell 9, 420–432 (2011).
Watanabe, R. et al. Gene expression profiles of cardiomyocytes in rat autoimmune myocarditis by DNA microarray and increase of regenerating gene family. Transl. Res. 152, 119–127 (2008).
Closa, D., Motoo, Y. & Iovanna, J.L. Pancreatitis-associated protein: from a lectin to an anti-inflammatory cytokine. World J. Gastroenterol. 13, 170–174 (2007).
Wilkinson, P.C. Cell locomotion and chemotaxis: basic concepts and methodological approaches. Methods 10, 74–81 (1996).
Gouwy, M. et al. Synergy between coproduced CC and CXC chemokines in monocyte chemotaxis through receptor-mediated events. Mol. Pharmacol. 74, 485–495 (2008).
Mortier, A., Van Damme, J. & Proost, P. Overview of the mechanisms regulating chemokine activity and availability. Immunol. Lett. 145, 2–9 (2012).
Benigni, F. et al. Six different cytokines that share GP130 as a receptor subunit, induce serum amyloid A and potentiate the induction of interleukin-6 and the activation of the hypothalamus-pituitary-adrenal axis by interleukin-1. Blood 87, 1851–1854 (1996).
Hintzen, C., Haan, C., Tuckermann, J.P., Heinrich, P.C. & Hermanns, H.M. Oncostatin M-induced and constitutive activation of the JAK2/STAT5/CIS pathway suppresses CCL1, but not CCL7 and CCL8, chemokine expression. J. Immunol. 181, 7341–7349 (2008).
Kiji, T. et al. Activation of regenerating gene Reg in rat and human hearts in response to acute stress. Am. J. Physiol. Heart Circ. Physiol. 289, H277–H284 (2005).
Iovanna, J., Orelle, B., Keim, V. & Dagorn, J.C. Messenger RNA sequence and expression of rat pancreatitis-associated protein, a lectin-related protein overexpressed during acute experimental pancreatitis. J. Biol. Chem. 266, 24664–24669 (1991).
Lai, Y. et al. The antimicrobial protein REG3A regulates keratinocyte proliferation and differentiation after skin injury. Immunity 37, 74–84 (2012).
Nishimune, H. et al. Reg-2 is a motoneuron neurotrophic factor and a signalling intermediate in the CNTF survival pathway. Nat. Cell Biol. 2, 906–914 (2000).
Livesey, F.J. et al. A Schwann cell mitogen accompanying regeneration of motor neurons. Nature 390, 614–618 (1997).
Namikawa, K., Okamoto, T., Suzuki, A., Konishi, H. & Kiyama, H. Pancreatitis-associated protein-III is a novel macrophage chemoattractant implicated in nerve regeneration. J. Neurosci. 26, 7460–7467 (2006).
Deten, A., Volz, H.C., Briest, W. & Zimmer, H.G. Cardiac cytokine expression is upregulated in the acute phase after myocardial infarction. Experimental studies in rats. Cardiovasc. Res. 55, 329–340 (2002).
Zidar, N., Jeruc, J., Balazic, J. & Stajer, D. Neutrophils in human myocardial infarction with rupture of the free wall. Cardiovasc. Pathol. 14, 247–250 (2005).
Cox, G., Crossley, J. & Xing, Z. Macrophage engulfment of apoptotic neutrophils contributes to the resolution of acute pulmonary inflammation in vivo. Am. J. Respir. Cell Mol. Biol. 12, 232–237 (1995).
Goren, I. et al. A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am. J. Pathol. 175, 132–147 (2009).
Gordy, C., Pua, H., Sempowski, G.D. & He, Y.W. Regulation of steady-state neutrophil homeostasis by macrophages. Blood 117, 618–629 (2011).
Hilfiker-Kleiner, D. et al. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ. Res. 95, 187–195 (2004).
Oshima, Y. et al. STAT3 mediates cardioprotection against ischemia/reperfusion injury through metallothionein induction in the heart. Cardiovasc. Res. 65, 428–435 (2005).
Haghikia, A., Stapel, B., Hoch, M. & Hilfiker-Kleiner, D. STAT3 and cardiac remodeling. Heart Fail. Rev. 16, 35–47 (2011).
Stross, C. et al. Oncostatin M receptor-mediated signal transduction is negatively regulated by SOCS3 through a receptor tyrosine-independent mechanism. J. Biol. Chem. 281, 8458–8468 (2006).
Chattopadhyay, S. et al. Interleukin-31 and oncostatin-M mediate distinct signaling reactions and response patterns in lung epithelial cells. J. Biol. Chem. 282, 3014–3026 (2007).
Kosmala, W., Przewlocka-Kosmala, M. & Mazurek, W. Proinflammatory cytokines and myocardial viability in patients after acute myocardial infarction. Int. J. Cardiol. 101, 449–456 (2005).
Jiang, B. & Liao, R. The paradoxical role of inflammation in cardiac repair and regeneration. J. Cardiovasc. Transl. Res. 3, 410–416 (2010).
Kempf, T., Zarbock, A., Vestweber, D. & Wollert, K.C. Anti-inflammatory mechanisms and therapeutic opportunities in myocardial infarct healing. J. Mol. Med. (Berl.) 90, 361–369 (2012).
Tanaka, M. et al. Targeted disruption of oncostatin M receptor results in altered hematopoiesis. Blood 102, 3154–3162 (2003).
Lieu, H.T. et al. Reg2 inactivation increases sensitivity to Fas hepatotoxicity and delays liver regeneration post-hepatectomy in mice. Hepatology 44, 1452–1464 (2006).
Ebelt, H. et al. Cellular cardiomyoplasty: improvement of left ventricular function correlates with the release of cardioactive cytokines. Stem Cells 25, 236–244 (2007).
Larson, A.C. et al. Self-gated cardiac cine MRI. Magn. Reson. Med. 51, 93–102 (2004).
Kubin, T. et al. Porcine aortic endothelial cells show little effects on smooth muscle cells but are potent stimulators of cardiomyocyte growth. Mol. Cell. Biochem. 242, 39–45 (2003).
Kubin, T. et al. Microvascular endothelial cells remodel cultured adult cardiomyocytes and increase their survival. Am. J. Physiol. 276, H2179–H2187 (1999).
Tofaris, G.K., Patterson, P.H., Jessen, K.R. & Mirsky, R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J. Neurosci. 22, 6696–6703 (2002).
Zamilpa, R. et al. CC chemokine receptor 5 deletion impairs macrophage activation and induces adverse remodeling following myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 300, H1418–H1426 (2011).
Boettger, T. et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J. Clin. Invest. 119, 2634–2647 (2009).
Neuhaus, P. et al. Reduced mobility of fibroblast growth factor (FGF)-deficient myoblasts might contribute to dystrophic changes in the musculature of FGF2/FGF6/mdx triple-mutant mice. Mol. Cell. Biol. 23, 6037–6048 (2003).
Elsässer, A. et al. A self-perpetuating vicious cycle of tissue damage in human hibernating myocardium. Mol. Cell. Biochem. 213, 17–28 (2000).
Wiggins, H. & Rappoport, J. An agarose spot assay for chemotactic invasion. Biotechniques 48, 121–124 (2010).
Virag, J.A. & Lust, R.M. Coronary artery ligation and intramyocardial injection in a murine model of infarction. J. Vis. Exp. 52, 2581 (2011).
Acknowledgements
We thank J. Wetzel, K. Richter, B. Matzke, U. Hofmann and S. Thomas for excellent technical assistance. We are indebted to A. Miyajima (University of Tokyo, Tokyo, Japan) and S. Hunt (University College London, London, UK) for the generous gifts of OSMR-deficient and Reg3β-deficient mice, respectively. This work was supported by the Foundation Leducq, the Excellence Initiative “Cardiopulmonary System,” the Cell and Gene Therapy Center (CGT) and the German Center for Cardiovascular Research (DZHK).
Author information
Authors and Affiliations
Contributions
H.L., J.P., P.G. and T.K. conducted experiments; Y.H. carried out experimental LAD ligations; V.P. and S.K. performed immunohistological analysis; J.M.A.-S. conducted data analyses; T.B. performed Genechip-based transcriptional profiling; A.W. performed and interpreted MRI analysis; H.W. supervised the project; M.R. provided biopsies of human ischemic cardiomyopathy samples; and H.L., J.P. and T.B. developed the concept, designed experimental studies, analyzed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 and Supplementary Tables 1–5 (PDF 13719 kb)
Supplementary Data Set
Statistical data for Supplementary Figures (XLSX 38 kb)
Rights and permissions
About this article
Cite this article
Lörchner, H., Pöling, J., Gajawada, P. et al. Myocardial healing requires Reg3β-dependent accumulation of macrophages in the ischemic heart. Nat Med 21, 353–362 (2015). https://doi.org/10.1038/nm.3816
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3816
This article is cited by
-
OSMR deficiency aggravates pressure overload-induced cardiac hypertrophy by modulating macrophages and OSM/LIFR/STAT3 signalling
Journal of Translational Medicine (2023)
-
Antimicrobial protein REG3A regulates glucose homeostasis and insulin resistance in obese diabetic mice
Communications Biology (2023)
-
Mechanisms of mucosal healing: treating inflammatory bowel disease without immunosuppression?
Nature Reviews Gastroenterology & Hepatology (2022)
-
Myeloid cell-specific ablation of Runx2 gene exacerbates post-infarct cardiac remodeling
Scientific Reports (2022)
-
A double-edged sword of immuno-microenvironment in cardiac homeostasis and injury repair
Signal Transduction and Targeted Therapy (2021)