Abstract
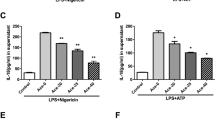
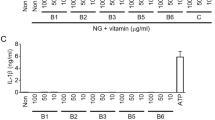
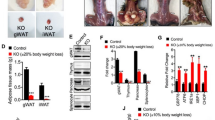
The ketone bodies β-hydroxybutyrate (BHB) and acetoacetate (AcAc) support mammalian survival during states of energy deficit by serving as alternative sources of ATP1. BHB levels are elevated by starvation, caloric restriction, high-intensity exercise, or the low-carbohydrate ketogenic diet2. Prolonged fasting reduces inflammation; however, the impact that ketones and other alternative metabolic fuels produced during energy deficits have on the innate immune response is unknown2,3,4,5,6. We report that BHB, but neither AcAc nor the structurally related short-chain fatty acids butyrate and acetate, suppresses activation of the NLRP3 inflammasome in response to urate crystals, ATP and lipotoxic fatty acids. BHB did not inhibit caspase-1 activation in response to pathogens that activate the NLR family, CARD domain containing 4 (NLRC4) or absent in melanoma 2 (AIM2) inflammasome and did not affect non-canonical caspase-11, inflammasome activation. Mechanistically, BHB inhibits the NLRP3 inflammasome by preventing K+ efflux and reducing ASC oligomerization and speck formation. The inhibitory effects of BHB on NLRP3 are not dependent on chirality or starvation-regulated mechanisms like AMP-activated protein kinase (AMPK), reactive oxygen species (ROS), autophagy or glycolytic inhibition. BHB blocks the NLRP3 inflammasome without undergoing oxidation in the TCA cycle, and independently of uncoupling protein-2 (UCP2), sirtuin-2 (SIRT2), the G protein–coupled receptor GPR109A or hydrocaboxylic acid receptor 2 (HCAR2). BHB reduces NLRP3 inflammasome–mediated interleukin (IL)-1β and IL-18 production in human monocytes. In vivo, BHB or a ketogenic diet attenuates caspase-1 activation and IL-1β secretion in mouse models of NLRP3-mediated diseases such as Muckle–Wells syndrome, familial cold autoinflammatory syndrome and urate crystal–induced peritonitis. Our findings suggest that the anti-inflammatory effects of caloric restriction or ketogenic diets may be linked to BHB-mediated inhibition of the NLRP3 inflammasome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Newman, J.C. & Verdin, E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 25, 42–52 (2014).
Cotter, D.G., Schugar, R.C. & Crawford, P.A. Ketone body metabolism and cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 304, H1060–H1076 (2013).
Shido, O., Nagasaka, T. & Watanabe, T. Blunted febrile response to intravenous endotoxin in starved rats. J. Appl. Physiol. 67, 963–969 (1989).
Johnson, J.B. et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic. Biol. Med. 42, 665–674 (2007).
Mercken, E.M. et al. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell 12, 645–651 (2013).
McGettrick, A.F. & O'Neill, L.A. How metabolism generates signals during innate immunity and inflammation. J. Biol. Chem. 288, 22893–22898 (2013).
Martinon, F., Mayor, A. & Tschopp, J. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27, 229–265 (2009).
Lamkanfi, M. & Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 157, 1013–1022 (2014).
Wen, H., Miao, E.A. & Ting, J.P. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity 39, 432–441 (2013).
Latz, E., Xiao, T.S. & Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13, 397–411 (2013).
Franchi, L., Eigenbrod, T., Muñoz-Planillo, R. & Nuñez, G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 10, 241–247 (2009).
Vandanmagsar, B. et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 17, 179–188 (2011).
Masters, S.L. et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol. 11, 897–904 (2010).
Heneka, M.T. et al. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678 (2013).
Martinon, F., Pétrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Wen, H. et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 12, 408–415 (2011).
Shaw, P.J. et al. Cutting edge: critical role for PYCARD/ASC in the development of experimental autoimmune encephalomyelitis. J. Immunol. 184, 4610–4614 (2010).
Youm, Y.H. et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 18, 519–532 (2013).
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Hagar, J.A., Powell, D.A., Aachoui, Y., Ernst, R.K. & Miao, E.A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013).
Shimazu, T. et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339, 211–214 (2013).
Laeger, T., Pöhland, R., Metges, C.C. & Kuhla, B. The ketone body β-hydroxybutyric acid influences agouti-related peptide expression via AMP-activated protein kinase in hypothalamic GT1–7 cells. J. Endocrinol. 213, 193–203 (2012).
Finn, P.F. & Dice, J.F. Ketone bodies stimulate chaperone-mediated autophagy. J. Biol. Chem. 280, 25864–25870 (2005).
Muñoz-Planillo, R. et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 (2013).
Taggart, A.K. et al. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 280, 26649–26652 (2005).
Singh, N. et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40, 128–139 (2014).
Cotter, D.G., Ercal, B., d'Avignon, D.A., Dietzen, D.J. & Crawford, P.A. Impact of peripheral ketolytic deficiency on hepatic ketogenesis and gluconeogenesis during the transition to birth. J. Biol. Chem. 288, 19739–19749 (2013).
Misawa, T. et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 14, 454–460 (2013).
Lutas, A. & Yellen, G. The ketogenic diet: metabolic influences on brain excitability and epilepsy. Trends Neurosci. 36, 32–40 (2013).
Lu, A. et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 156, 1193–1206 (2014).
Yu, J.W. et al. Cryopyrin and pyrin activate caspase-1, but not NF-κB, via ASC oligomerization. Cell Death Differ. 13, 236–249 (2006).
Demento, S.L., Siefert, A.L., Bandyopadhyay, A., Sharp, F.A. & Fahmy, T.M. Pathogen-associated molecular patterns on biomaterials: a paradigm for engineering new vaccines. Trends Biotechnol. 29, 294–306 (2011).
Martinon, F., Pétrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Brydges, S.D. et al. Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity. Immunity 30, 875–887 (2009).
Anand, P.K. et al. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature 488, 389–393 (2012).
Acknowledgements
We thank M. Koch, Y. Kui, P. Chang and D. Albarado for technical assistance, and V. M. Dixit (Genentech) and R. Medzhitov (Yale School of Medicine) for helpful discussions and for providing knockout mice. Salmonella typhimurium and Francisella tularensis (U112) were provided by D. Monack (Stanford University School of Medicine) and J. Teale (University of Texas at San Antonio). M.B. and A.B. were supported by the National Institute on Aging–Intramural Research Program. D.D'A. was supported by the Office of Naval Research (ONR) Grant N000141310062. V.D.D. was supported in part by the grants from National Institutes of Health (AG043608, AG31797, DK090556 and AI105097).
Author information
Authors and Affiliations
Contributions
Y. H.Y. and K.Y.N. designed and conducted the majority of in vitro and all in vivo experiments, analyzed and interpreted the data, and participated in writing the manuscript. R.W.G. participated in design and conduct of inflammasome activation experiments. E.L.G. performed ASC speck and neutrophil assays. M.B. and A.B. performed the human monocytes experiments. D.K. and T.M.F. synthesized the BHB–nanolipogels and conducted control experiments to determine the dose response. D.D'A. formulated the ketone diester diet. N.P. conducted the ICP-MS experiments to determine K+ efflux. C.L. and T.D.K. conducted the F. tularensis and S. typhimurium infection experiments. T.L.H. designed the experiments and provided essential reagents for experiments involving mitochondrial ROS and UCP2. P.A.C. generated the macrophage-specific, Scot-deficient mice and contributed to experiment design. S.K. and E.A. designed and conducted the ASC oligomerization experiments. V.D.D. conceived and supervised the project, interpreted the data, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 5425 kb)
Rights and permissions
About this article
Cite this article
Youm, YH., Nguyen, K., Grant, R. et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat Med 21, 263–269 (2015). https://doi.org/10.1038/nm.3804
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3804
This article is cited by
-
Coenzyme Q10 mitigates macrophage mediated inflammation in heart following myocardial infarction via the NLRP3/IL1β pathway
BMC Cardiovascular Disorders (2024)
-
AMPKα2 regulates fasting-induced hyperketonemia by suppressing SCOT ubiquitination and degradation
Scientific Reports (2024)
-
Ketosis prevents abdominal aortic aneurysm rupture through C–C chemokine receptor type 2 downregulation and enhanced extracellular matrix balance
Scientific Reports (2024)
-
Cytosolic mtDNA–cGAS–STING axis contributes to sepsis-induced acute kidney injury via activating the NLRP3 inflammasome
Clinical and Experimental Nephrology (2024)
-
Immune Response Gene-1 [IRG1]/itaconate protect against multi-organ injury via inhibiting gasdermin D-mediated pyroptosis and inflammatory response
Inflammopharmacology (2024)