Abstract
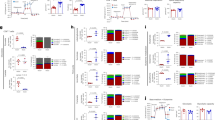
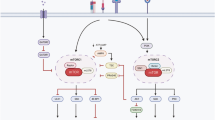
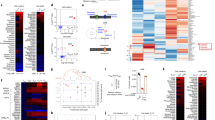
CD4+ T cells differentiate into memory T cells that protect the host from subsequent infection. In contrast, autoreactive memory CD4+ T cells harm the body by persisting in the tissues. The underlying pathways controlling the maintenance of memory CD4+ T cells remain undefined. We show here that memory CD4+ T cell survival is impaired in the absence of the Notch signaling protein known as recombination signal binding protein for immunoglobulin κ J region (Rbpj). Treatment of mice with a Notch inhibitor reduced memory CD4+ T cell numbers and prevented the recurrent induction of experimental autoimmune encephalomyelitis. Rbpj-deficient CD4+ memory T cells exhibit reduced glucose uptake due to impaired AKT phosphorylation, resulting in low Glut1 expression. Treating mice with pyruvic acid, which bypasses glucose uptake and supplies the metabolite downstream of glucose uptake, inhibited the decrease of autoimmune memory CD4+ T cells in the absence of Notch signaling, suggesting memory CD4+ T cell survival relies on glucose metabolism. Together, these data define a central role for Notch signaling in maintaining memory CD4+ T cells through the regulation of glucose uptake.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Castellino, F. & Germain, R.N. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu. Rev. Immunol. 24, 519–540 (2006).
Reiner, S.L. Decision making during the conception and career of CD4+ T cells. Nat. Rev. Immunol. 9, 81–82 (2009).
Zhu, J., Yamane, H. & Paul, W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 28, 445–489 (2010).
Williams, M.A. & Bevan, M.J. Effector and memory CTL differentiation. Annu. Rev. Immunol. 25, 171–192 (2007).
Weng, N.P., Araki, Y. & Subedi, K. The molecular basis of the memory T cell response: differential gene expression and its epigenetic regulation. Nat. Rev. Immunol. 12, 306–315 (2012).
Bevan, M.J. Memory T cells as an occupying force. Eur. J. Immunol. 41, 1192–1195 (2011).
Pearce, E.L. et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009).
Araki, K. et al. mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112 (2009).
Yuan, J.S., Kousis, P.C., Suliman, S., Visan, I. & Guidos, C.J. Functions of notch signaling in the immune system: consensus and controversies. Annu. Rev. Immunol. 28, 343–365 (2010).
Maillard, I., Fang, T. & Pear, W.S. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu. Rev. Immunol. 23, 945–974 (2005).
Radtke, F., Fasnacht, N. & Macdonald, H.R. Notch signaling in the immune system. Immunity 32, 14–27 (2010).
Wolfer, A. et al. Inactivation of Notch 1 in immature thymocytes does not perturb CD4 or CD8 T cell development. Nat. Immunol. 2, 235–241 (2001).
Maekawa, Y. et al. Notch2 integrates signaling by the transcription factors RBP-J and CREB1 to promote T cell cytotoxicity. Nat. Immunol. 9, 1140–1147 (2008).
Alam, M.S. et al. Notch signaling drives IL-22 secretion in CD4+ T cells by stimulating the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 107, 5943–5948 (2010).
Amsen, D. et al. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity 27, 89–99 (2007).
Tu, L. et al. Notch signaling is an important regulator of type 2 immunity. J. Exp. Med. 202, 1037–1042 (2005).
Amsen, D. et al. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell 117, 515–526 (2004).
Tanaka, S. et al. The interleukin-4 enhancer CNS-2 is regulated by Notch signals and controls initial expression in NKT cells and memory-type CD4 T cells. Immunity 24, 689–701 (2006).
Helbig, C. et al. Notch controls the magnitude of T helper cell responses by promoting cellular longevity. Proc. Natl. Acad. Sci. USA 109, 9041–9046 (2012).
Tokoyoda, K. et al. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity 30, 721–730 (2009).
Sadick, M.D. et al. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell–dependent, interferon γ-independent mechanism. J. Exp. Med. 171, 115–127 (1990).
Thorens, B. & Mueckler, M. Glucose transporters in the 21st century. Am. J. Physiol. Endocrinol. Metab. 298, E141–E145 (2010).
Maciver, N.J. et al. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J. Leukoc. Biol. 84, 949–957 (2008).
Tremblay, F., Dubois, M.J. & Marette, A. Regulation of GLUT4 traffic and function by insulin and contraction in skeletal muscle. Front. Biosci. 8, d1072–d1084 (2003).
Ishiki, M. & Klip, A. Minireview: recent developments in the regulation of glucose transporter-4 traffic: new signals, locations, and partners. Endocrinology 146, 5071–5078 (2005).
Youngblood, B., Davis, C.W. & Ahmed, R. Making memories that last a lifetime: heritable functions of self-renewing memory CD8 T cells. Int. Immunol. 22, 797–803 (2010).
Prlic, M., Williams, M.A. & Bevan, M.J. Requirements for CD8 T-cell priming, memory generation and maintenance. Curr. Opin. Immunol. 19, 315–319 (2007).
van Leeuwen, E.M., Sprent, J. & Surh, C.D. Generation and maintenance of memory CD4+ T cells. Curr. Opin. Immunol. 21, 167–172 (2009).
Pepper, M. & Jenkins, M.K. Origins of CD4+ effector and central memory T cells. Nat. Immunol. 12, 467–471 (2011).
Prlic, M. & Bevan, M.J. Immunology: A metabolic switch to memory. Nature 460, 41–42 (2009).
Ciofani, M. & Zuniga-Pflucker, J.C. Notch promotes survival of pre-T cells at the β-selection checkpoint by regulating cellular metabolism. Nat. Immunol. 6, 881–888 (2005).
Amsen, D., Antov, A. & Flavell, R.A. The different faces of Notch in T-helper-cell differentiation. Nat. Rev. Immunol. 9, 116–124 (2009).
Minter, L.M. et al. Inhibitors of γ-secretase block in vivo and in vitro T helper type 1 polarization by preventing Notch upregulation of Tbx21. Nat. Immunol. 6, 680–688 (2005).
Sandy, A.R. et al. T cell–specific notch inhibition blocks graft-versus-host disease by inducing a hyporesponsive program in alloreactive CD4+ and CD8+ T cells. J. Immunol. 190, 5818–5828 (2013).
Kryczek, I. et al. Human TH17 cells are long-lived effector memory cells. Sci Transl. Med. 3, 104ra100 (2011).
Han, H. et al. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int. Immunol. 14, 637–645 (2002).
Saito, T. et al. Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity 18, 675–685 (2003).
Hozumi, K. et al. Delta-like 1 is necessary for the generation of marginal zone B cells but not T cells in vivo. Nat. Immunol. 5, 638–644 (2004).
Burchill, M.A. et al. Distinct effects of STAT5 activation on CD4+ and CD8+ T cell homeostasis: development of CD4+ CD25+ regulatory T cells versus CD8+ memory T cells. J. Immunol. 171, 5853–5864 (2003).
Maekawa, Y. et al. Delta1-Notch3 interactions bias the functional differentiation of activated CD4+ T cells. Immunity 19, 549–559 (2003).
Acknowledgements
We thank S. Chiba (University of Tsukuba), K. Tanigaki (Shiga Medical Center Research Institute), T. Honjo (Kyoto University), M. Kubo (Tokyo University of Science), M. Farrar (University of Minnesota) and Y. Itoh (Shiga Medical University) for providing mice; T. Azuma (Tokyo Science University) for antibodies; and C. Kinouchi and C. Tomari for technical and editorial assistance. This work was supported by a Grant-in-Aid for Young Scientists (S) from the Japan Society for the Promotion of Science and by a Grant-in-Aid for Scientific Research on Innovative Areas ('Dying Codes') from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Author information
Authors and Affiliations
Contributions
Y.M. conducted all of the experiments, analyzed data and wrote the manuscript; C.I. and S.T. contributed to the in vivo T cell transfer studies; K.H. established the Dll1-deficient mouse; H.Y. prepared anti-Dll1 and Dll4 antibodies; and K.Y. supervised the project, analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–14 and Supplementary Table 1 (PDF 1565 kb)
Rights and permissions
About this article
Cite this article
Maekawa, Y., Ishifune, C., Tsukumo, Si. et al. Notch controls the survival of memory CD4+ T cells by regulating glucose uptake. Nat Med 21, 55–61 (2015). https://doi.org/10.1038/nm.3758
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3758
This article is cited by
-
New insights into the stemness of adoptively transferred T cells by γc family cytokines
Cell Communication and Signaling (2023)
-
Skin T cells maintain their diversity and functionality in the elderly
Communications Biology (2021)
-
Metabolic signaling in T cells
Cell Research (2020)
-
Tissue-resident memory T cells and their biological characteristics in the recurrence of inflammatory skin disorders
Cellular & Molecular Immunology (2020)
-
A dendritic cell-based systemic vaccine induces long-lived lung-resident memory Th17 cells and ameliorates pulmonary mycosis
Mucosal Immunology (2019)