Abstract
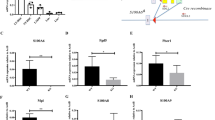
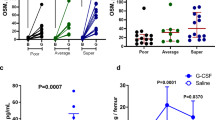
Multiple bone marrow stromal cell types have been identified as hematopoietic stem cell (HSC)-regulating niche cells1,2,3,4,5,6,7. However, whether HSC progeny can serve directly as HSC niche cells has not previously been shown. Here we report a dichotomous role of megakaryocytes (MKs) in both maintaining HSC quiescence during homeostasis and promoting HSC regeneration after chemotherapeutic stress. We show that MKs are physically associated with HSCs in the bone marrow of mice and that MK ablation led to activation of quiescent HSCs and increased HSC proliferation. RNA sequencing (RNA-seq) analysis revealed that transforming growth factor β1 (encoded by Tgfb1) is expressed at higher levels in MKs as compared to other stromal niche cells. MK ablation led to reduced levels of biologically active TGF-β1 protein in the bone marrow and nuclear-localized phosphorylated SMAD2/3 (pSMAD2/3) in HSCs, suggesting that MKs maintain HSC quiescence through TGF-β–SMAD signaling8,9. Indeed, TGF-β1 injection into mice in which MKs had been ablated restored HSC quiescence, and conditional deletion of Tgfb1 in MKs increased HSC activation and proliferation. These data demonstrate that TGF-β1 is a dominant signal emanating from MKs that maintains HSC quiescence. However, under conditions of chemotherapeutic challenge, MK ablation resulted in a severe defect in HSC expansion. In response to stress, fibroblast growth factor 1 (FGF1) signaling from MKs transiently dominates over TGF-β inhibitory signaling to stimulate HSC expansion10,11. Overall, these observations demonstrate that MKs serve as HSC-derived niche cells to dynamically regulate HSC function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zhang, J. et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425, 836–841 (2003).
Calvi, L.M. et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425, 841–846 (2003).
Kiel, M.J., Yilmaz, O.H., Iwashita, T., Terhorst, C. & Morrison, S.J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005).
Sugiyama, T., Kohara, H., Noda, M. & Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25, 977–988 (2006).
Méndez-Ferrer, S. et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 (2010).
Ding, L., Saunders, T.L., Enikolopov, G. & Morrison, S.J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462 (2012).
Kunisaki, Y. et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 23, 3865–3873 (2013).
Yamazaki, S. et al. TGF-β as a candidate bone marrow niche signal to induce hematopoietic stem cell hibernation. Blood 113, 1250–1256 (2009).
Yamazaki, S. et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147, 1146–1158 (2011).
Zhao, M. et al. FGF signaling facilitates postinjury recovery of mouse hematopoietic system. Blood 120, 1831–1842 (2012).
Itkin, T. et al. FGF-2 expands murine hematopoietic stem and progenitor cells via proliferation of stromal cells, c-Kit activation, and CXCL12 down-regulation. Blood 120, 1843–1855 (2012).
Li, L. & Clevers, H. Coexistence of quiescent and active adult stem cells in mammals. Science 327, 542–545 (2010).
Winkler, I.G. et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 116, 4815–4828 (2010).
Chow, A. et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J. Exp. Med. 208, 261–271 (2011).
Chow, A. et al. CD169+ macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat. Med. 19, 429–436 (2013).
Dominici, M. et al. Restoration and reversible expansion of the osteoblastic hematopoietic stem cell niche after marrow radioablation. Blood 114, 2333–2343 (2009).
Olson, T.S. et al. Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood 121, 5238–5249 (2013).
Heazlewood, S.Y. et al. Megakaryocytes co-localise with hemopoietic stem cells and release cytokines that up-regulate stem cell proliferation. Stem Cell Res. 11, 782–792 (2013).
Deutsch, V.R. & Tomer, A. Megakaryocyte development and platelet production. Br. J. Haematol. 134, 453–466 (2006).
Xie, Y. et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature 457, 97–101 (2009).
Tiedt, R., Schomber, T., Hao-Shen, H. & Skoda, R.C. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood 109, 1503–1506 (2007).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Buch, T. et al. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat. Methods 2, 419–426 (2005).
Yang, L. et al. Identification of Lin−Sca1+kit+CD34+Flt3− short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood 105, 2717–2723 (2005).
Chen, C.Z. et al. Identification of endoglin as a functional marker that defines long-term repopulating hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 99, 15468–15473 (2002).
Bruns, I. et al. Megakaryocytes regulate hematopoietic stem cell quiescence through Cxcl4 secretion. Nat. Med. 10.1038/nm.3707 (19 October 2014).
Longley, D.B., Harkin, D.P. & Johnston, P.G. 5-fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer 3, 330–338 (2003).
Essers, M.A. et al. IFNα activates dormant haematopoietic stem cells in vivo. Nature 458, 904–908 (2009).
Elmasri, H. et al. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J. 23, 3865–3873 (2009).
Avecilla, S.T. et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat. Med. 10, 64–71 (2004).
Brenet, F., Kermani, P., Spektor, R., Rafii, S. & Scandura, J.M. TGFβ restores hematopoietic homeostasis after myelosuppressive chemotherapy. J. Exp. Med. 210, 623–639 (2013).
de Haan, G. et al. In vitro generation of long-term repopulating hematopoietic stem cells by fibroblast growth factor-1. Dev. Cell 4, 241–251 (2003).
Zhang, C.C. et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat. Med. 12, 240–245 (2006).
Perry, J.M. et al. Cooperation between both Wnt/β-catenin and PTEN/PI3K/Akt signaling promotes primitive hematopoietic stem cell self-renewal and expansion. Genes Dev. 25, 1928–1942 (2011).
Calaminus, S.D. et al. Lineage tracing of Pf4-Cre marks hematopoietic stem cells and their progeny. PLoS ONE 7, e51361 (2012).
Xu, X., Qiao, W., Li, C. & Deng, C.X. Generation of Fgfr1 conditional knockout mice. Genesis 32, 85–86 (2002).
Azhar, M. et al. Generation of mice with a conditional allele for transforming growth factor β1 gene. Genesis 47, 423–431 (2009).
Meyer, A. et al. Platelet TGF-β1 contributions to plasma TGF-β1, cardiac fibrosis, and systolic dysfunction in a mouse model of pressure overload. Blood 119, 1064–1074 (2012).
Sugimura, R. et al. Noncanonical Wnt signaling maintains hematopoietic stem cells in the niche. Cell 150, 351–365 (2012).
Krause, D.S. et al. Differential regulation of myeloid leukemias by the bone marrow microenvironment. Nat. Med. 19, 1513–1517 (2013).
Acknowledgements
We are grateful to members of the Li lab for scientific discussion and manuscript critical reading, M. Hembree, K. Zapien, D. Dukes and B. Lewis for technical support and K. Tannen for manuscript editing and proofreading. We thank H. Lin (Massachusetts General Hospital), who generously provided the CAGA-luc plasmid. We thank C. Deng (US National Institutes of Health) for providing Fgfr1flox/flox mice. This work was funded by the Stowers Institute for Medical Research.
Author information
Authors and Affiliations
Contributions
M.Z. performed experiments, analyzed data and wrote the paper. J.M.P. contributed to HSC culture studies and edited the manuscript. H.M. contributed to part of the mouse work. A.V., P.Q. and X.C.H. performed RNA-seq. J.A. shared reagents. L.L. directed the overall project and co-wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 (PDF 1717 kb)
Rights and permissions
About this article
Cite this article
Zhao, M., Perry, J., Marshall, H. et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med 20, 1321–1326 (2014). https://doi.org/10.1038/nm.3706
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3706
This article is cited by
-
Endothelial-derived small extracellular vesicles support B-cell acute lymphoblastic leukemia development
Cellular Oncology (2024)
-
Cellular niches for hematopoietic stem cells in bone marrow under normal and malignant conditions
Inflammation and Regeneration (2023)
-
The spatiotemporal heterogeneity of the biophysical microenvironment during hematopoietic stem cell development: from embryo to adult
Stem Cell Research & Therapy (2023)
-
The roles of bone remodeling in normal hematopoiesis and age-related hematological malignancies
Bone Research (2023)
-
AGO2 silences mobile transposons in the nucleus of quiescent cells
Nature Structural & Molecular Biology (2023)