Abstract
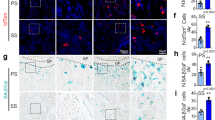
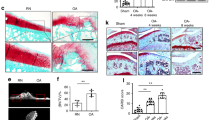
Osteogenesis during bone modeling and remodeling is coupled with angiogenesis. A recent study showed that a specific vessel subtype, strongly positive for CD31 and endomucin (CD31hiEmcnhi), couples angiogenesis and osteogenesis. Here, we found that platelet-derived growth factor-BB (PDGF-BB) secreted by preosteoclasts induces CD31hiEmcnhi vessel formation during bone modeling and remodeling. Mice with depletion of PDGF-BB in the tartrate-resistant acid phosphatase–positive cell lineage show significantly lower trabecular and cortical bone mass, serum and bone marrow PDGF-BB concentrations, and fewer CD31hiEmcnhi vessels compared to wild-type mice. In the ovariectomy (OVX)-induced osteoporotic mouse model, serum and bone marrow levels of PDGF-BB and numbers of CD31hiEmcnhi vessels are significantly lower compared to sham-operated controls. Treatment with exogenous PDGF-BB or inhibition of cathepsin K to increase the number of preosteoclasts, and thus the endogenous levels of PDGF-BB, increases CD31hiEmcnhi vessel number and stimulates bone formation in OVX mice. Thus, pharmacotherapies that increase PDGF-BB secretion from preosteoclasts offer a new therapeutic target for treating osteoporosis by promoting angiogenesis and thus bone formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Seeman, E. Bone modeling and remodeling. Crit. Rev. Eukaryot. Gene Expr. 19, 219–233 (2009).
Teti, A. Bone development: overview of bone cells and signaling. Curr. Osteoporos. Rep. 9, 264–273 (2011).
Eriksen, E.F. Cellular mechanisms of bone remodeling. Rev. Endocr. Metab. Disord. 11, 219–227 (2010).
Zaidi, M. Skeletal remodeling in health and disease. Nat. Med. 13, 791–801 (2007).
Portal-Núñez, S., Lozano, D. & Esbrit, P. Role of angiogenesis on bone formation. Histol. Histopathol. 27, 559–566 (2012).
Brandi, M.L. & Collin-Osdoby, P. Vascular biology and the skeleton. J. Bone Miner. Res. 21, 183–192 (2006).
Kusumbe, A.P., Ramasamy, S.K. & Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323–328 (2014).
Ramasamy, S.K., Kusumbe, A.P., Wang, L. & Adams, R.H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 507, 376–380 (2014).
Sims, N.A. & Martin, T.J. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. BoneKEy Rep. 3, 481 (2014).
Del Fattore, A., Teti, A. & Rucci, N. Bone cells and the mechanisms of bone remodelling. Front. Biosci. (Elite Ed.) 4, 2302–2321 (2012).
Ishii, M. & Saeki, Y. Osteoclast cell fusion: mechanisms and molecules. Mod. Rheumatol. 18, 220–227 (2008).
Tang, Y. et al. TGF-β1–induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 15, 757–765 (2009).
Xian, L. et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat. Med. 18, 1095–1101 (2012).
Henriksen, K., Karsdal, M.A. & Martin, T.J. Osteoclast-derived coupling factors in bone remodeling. Calcif. Tissue Int. 94, 88–97 (2014).
Del Fattore, A. et al. Clinical, genetic, and cellular analysis of 49 osteopetrotic patients: implications for diagnosis and treatment. J. Med. Genet. 43, 315–325 (2006).
Lee, S.H. et al. v-ATPase V0 subunit d2–deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat. Med. 12, 1403–1409 (2006).
Henriksen, K. et al. A specific subtype of osteoclasts secretes factors inducing nodule formation by osteoblasts. Bone 51, 353–361 (2012).
Baroukh, B., Cherruau, M., Dobigny, C., Guez, D. & Saffar, J.L. Osteoclasts differentiate from resident precursors in an in vivo model of synchronized resorption: a temporal and spatial study in rats. Bone 27, 627–634 (2000).
Ochareon, P. & Herring, S.W. Cell replication in craniofacial periosteum: appositional vs. resorptive sites. J. Anat. 218, 285–297 (2011).
Chang, M.K. et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J. Immunol. 181, 1232–1244 (2008).
Alexander, K.A. et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J. Bone Miner. Res. 26, 1517–1532 (2011).
Choi, I.H., Chung, C.Y., Cho, T.J. & Yoo, W.J. Angiogenesis and mineralization during distraction osteogenesis. J. Korean Med. Sci. 17, 435–447 (2002).
Percival, C.J. & Richtsmeier, J.T. Angiogenesis and intramembranous osteogenesis. Dev. Dyn. 242, 909–922 (2013).
Chim, S.M. et al. Angiogenic factors in bone local environment. Cytokine Growth Factor Rev. 24, 297–310 (2013).
Pazzaglia, U.E. et al. Morphometric analysis of the canal system of cortical bone: An experimental study in the rabbit femur carried out with standard histology and micro-CT. Anat. Histol. Embryol. 39, 17–26 (2010).
Parfitt, A.M. The mechanism of coupling: a role for the vasculature. Bone 26, 319–323 (2000).
Carmeliet, P. & Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307 (2011).
Chen, W.C. et al. Cellular kinetics of perivascular MSC precursors. Stem Cells Int. 2013, 983059 (2013).
Bronckaers, A. et al. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther. 143, 181–196 (2014).
Nassiri, S.M. & Rahbarghazi, R. Interactions of mesenchymal stem cells with endothelial cells. Stem Cells Dev. 23, 319–332 (2014).
Wang, H. et al. Over-expression of PDGFR-β promotes PDGF-induced proliferation, migration, and angiogenesis of EPCs through PI3K/Akt signaling pathway. PLoS ONE 7, e30503 (2012).
Fiedler, J., Etzel, N. & Brenner, R.E. To go or not to go: Migration of human mesenchymal progenitor cells stimulated by isoforms of PDGF. J. Cell. Biochem. 93, 990–998 (2004).
Caplan, A.I. & Correa, D. PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs. J. Orthop. Res. 29, 1795–1803 (2011).
Kreja, L. et al. Non-resorbing osteoclasts induce migration and osteogenic differentiation of mesenchymal stem cells. J. Cell. Biochem. 109, 347–355 (2010).
Sanchez-Fernandez, M.A., Gallois, A., Riedl, T., Jurdic, P. & Hoflack, B. Osteoclasts control osteoblast chemotaxis via PDGF-BB/PDGF receptor β signaling. PLoS ONE 3, e3537 (2008).
Kubota, K., Sakikawa, C., Katsumata, M., Nakamura, T. & Wakabayashi, K. Platelet-derived growth factor BB secreted from osteoclasts acts as an osteoblastogenesis inhibitory factor. J. Bone Miner. Res. 17, 257–265 (2002).
Sakagami, N. et al. Reduced osteoblastic population and defective mineralization in osteopetrotic (op/op) mice. Micron 36, 688–695 (2005).
Hellström, M., Kalen, M., Lindahl, P., Abramsson, A. & Betsholtz, C. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126, 3047–3055 (1999).
Gelb, B.D., Shi, G.P., Chapman, H.A. & Desnick, R.J. Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science 273, 1236–1238 (1996).
Chen, W. et al. Novel pycnodysostosis mouse model uncovers cathepsin K function as a potential regulator of osteoclast apoptosis and senescence. Hum. Mol. Genet. 16, 410–423 (2007).
Brixen, K. et al. Bone density, turnover, and estimated strength in postmenopausal women treated with odanacatib: a randomized trial. J. Clin. Endocrinol. Metab. 98, 571–580 (2013).
Cusick, T. et al. Odanacatib treatment increases hip bone mass and cortical thickness by preserving endocortical bone formation and stimulating periosteal bone formation in the ovariectomized adult rhesus monkey. J. Bone Miner. Res. 27, 524–537 (2012).
Xiang, A. et al. Changes in micro-CT 3D bone parameters reflect effects of a potent cathepsin K inhibitor (SB-553484) on bone resorption and cortical bone formation in ovariectomized mice. Bone 40, 1231–1237 (2007).
Palmer, J.T. et al. Design and synthesis of tri-ring P3 benzamide–containing aminonitriles as potent, selective, orally effective inhibitors of cathepsin K. J. Med. Chem. 48, 7520–7534 (2005).
Zhao, X. & Guan, J.L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv. Drug Deliv. Rev. 63, 610–615 (2011).
Ryu, J. et al. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J. 25, 5840–5851 (2006).
Pederson, L., Ruan, M., Westendorf, J.J., Khosla, S. & Oursler, M.J. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc. Natl. Acad. Sci. USA 105, 20764–20769 (2008).
Lotinun, S. et al. Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation. J. Clin. Invest. 123, 666–681 (2013).
Roche, B. et al. Parathyroid hormone 1–84 targets bone vascular structure and perfusion in mice: impacts of its administration regimen and of ovariectomy. J. Bone Miner. Res. 29, 1608–1618 (2014).
Zhao, Q. et al. Mice with increased angiogenesis and osteogenesis due to conditional activation of HIF pathway in osteoblasts are protected from ovariectomy induced bone loss. Bone 50, 763–770 (2012).
Shih, T.T. et al. Correlation of MR lumbar spine bone marrow perfusion with bone mineral density in female subjects. Radiology 233, 121–128 (2004).
Seeman, E. & Delmas, P.D. Bone quality–the material and structural basis of bone strength and fragility. N. Engl. J. Med. 354, 2250–2261 (2006).
Mackie, E.J., Tatarczuch, L. & Mirams, M. The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification. J. Endocrinol. 211, 109–121 (2011).
Chatani, M., Takano, Y. & Kudo, A. Osteoclasts in bone modeling, as revealed by in vivo imaging, are essential for organogenesis in fish. Dev. Biol. 360, 96–109 (2011).
Witten, P.E. & Huysseune, A. A comparative view on mechanisms and functions of skeletal remodelling in teleost fish, with special emphasis on osteoclasts and their function. Biol. Rev. Camb. Philos. Soc. 84, 315–346 (2009).
Seeman, E. The periosteum - a surface for all seasons. Osteoporos. Int. 18, 123–128 (2007).
Friedlaender, G.E., Lin, S., Solchaga, L.A., Snel, L.B. & Lynch, S.E. The role of recombinant human platelet-derived growth factor-BB (rhPDGF-BB) in orthopaedic bone repair and regeneration. Curr. Pharm. Des. 19, 3384–3390 (2013).
Graham, S. et al. Investigating the role of PDGF as a potential drug therapy in bone formation and fracture healing. Expert Opin. Investig. Drugs 18, 1633–1654 (2009).
Costa, A.G., Cusano, N.E., Silva, B.C., Cremers, S. & Bilezikian, J.P. Cathepsin K: its skeletal actions and role as a therapeutic target in osteoporosis. Nat. Rev. Rheumatol. 7, 447–456 (2011).
Boonen, S., Rosenberg, E., Claessens, F., Vanderschueren, D. & Papapoulos, S. Inhibition of cathepsin K for treatment of osteoporosis. Curr. Osteoporos. Rep. 10, 73–79 (2012).
Dossa, T. et al. Osteoclast-specific inactivation of the integrin-linked kinase (ILK) inhibits bone resorption. J. Cell. Biochem. 110, 960–967 (2010).
Saftig, P. et al. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K–deficient mice. Proc. Natl. Acad. Sci. USA 95, 13453–13458 (1998).
Wu, X. et al. Inhibition of Sca-1-positive skeletal stem cell recruitment by alendronate blunts the anabolic effects of parathyroid hormone on bone remodeling. Cell Stem Cell 7, 571–580 (2010).
Zhen, G. et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 19, 704–712 (2013).
Duvall, C.L., Taylor, W.R., Weiss, D. & Guldberg, R.E. Quantitative microcomputed tomography analysis of collateral vessel development after ischemic injury. Am. J. Physiol. Heart Circ. Physiol. 287, H302–H310 (2004).
Wang, Y. et al. The hypoxia-inducible factor α pathway couples angiogenesis to osteogenesis during skeletal development. J. Clin. Invest. 117, 1616–1626 (2007).
Qiu, T. et al. TGF-β type II receptor phosphorylates PTH receptor to integrate bone remodelling signalling. Nat. Cell Biol. 12, 224–234 (2010).
Acknowledgements
This research was supported by US National Institutes of Health grants DK 057501 and AR 063943 (to X.C.), China National Funds for Distinguished Young Scientists grant 81125006 (to X.L.) and the Merck Investigator-Initiated Studies Program.
Author information
Authors and Affiliations
Contributions
H.X. conceived the ideas for experimental designs, conducted the majority of the experiments, analyzed data and prepared the manuscript. Z.C., L.W., Z.X. and Y.H. maintained mice and collected tissue samples, performed microcomputed tomography analyses, conducted immunohistochemistry and immunofluorescence, conducted cell culture and western blot experiments, and helped with manuscript preparation. L. Xian, C.L., L. Xie and W.C. maintained mice and helped with flow cytometry, cell culture and transwell migration assay. J.C., M.W., G.Z., Q.B., B.Y. and M.P. provided suggestions for the project and critically reviewed the manuscript. T.Q. performed confocal imaging. L.T.D. and J.J.W. provided mouse models. X.L. and E.L. participated in experimental design and helped compose the manuscript. X.C. developed the concept, supervised the project, conceived the experiments and wrote most of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
M.P. and L.T.D. are employees of Merck & Co. They own stocks and stock options from this company.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8. (PDF 1227 kb)
Rights and permissions
About this article
Cite this article
Xie, H., Cui, Z., Wang, L. et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med 20, 1270–1278 (2014). https://doi.org/10.1038/nm.3668
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3668
This article is cited by
-
Bone-targeting engineered small extracellular vesicles carrying anti-miR-6359-CGGGAGC prevent valproic acid-induced bone loss
Signal Transduction and Targeted Therapy (2024)
-
Age-related secretion of grancalcin by macrophages induces skeletal stem/progenitor cell senescence during fracture healing
Bone Research (2024)
-
Combination effect of Chinese kidney-tonifying granules and platelet-rich plasma gels on enhancing bone healing in rat models with femur defects
Journal of Orthopaedic Surgery and Research (2023)
-
Network pharmacology-based mechanism prediction and pharmacological validation of Bushenhuoxue formula attenuating postmenopausal osteoporosis in ovariectomized mice
Journal of Orthopaedic Surgery and Research (2023)
-
Preosteoclast plays a pathogenic role in syndesmophyte formation of ankylosing spondylitis through the secreted PDGFB — GRB2/ERK/RUNX2 pathway
Arthritis Research & Therapy (2023)