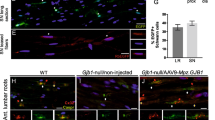
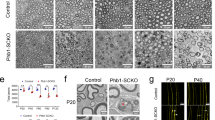
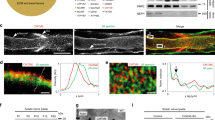
Abstract
Duplication of the gene encoding the peripheral myelin protein of 22 kDa (PMP22) underlies the most common inherited neuropathy, Charcot-Marie-Tooth 1A (CMT1A)1,2,3, a disease without a known cure4,5,6. Although demyelination represents a characteristic feature, the clinical phenotype of CMT1A is determined by the degree of axonal loss, and patients suffer from progressive muscle weakness and impaired sensation4,7. CMT1A disease manifests within the first two decades of life8,9, and walking disabilities, foot deformities and electrophysiological abnormalities are already present in childhood7,8,9,10,11. Here, we show in Pmp22-transgenic rodent models of CMT1A that Schwann cells acquire a persistent differentiation defect during early postnatal development, caused by imbalanced activity of the PI3K-Akt and the Mek-Erk signaling pathways. We demonstrate that enhanced PI3K-Akt signaling by axonally overexpressed neuregulin-1 (NRG1) type I drives diseased Schwann cells toward differentiation and preserves peripheral nerve axons. Notably, in a preclinical experimental therapy using a CMT1A rat model, when treatment is restricted to early postnatal development, soluble NRG1 effectively overcomes impaired peripheral nerve development and restores axon survival into adulthood. Our findings suggest a model in which Schwann cell differentiation within a limited time window is crucial for the long-term maintenance of axonal support.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sereda, M. et al. A transgenic rat model of Charcot-Marie-Tooth disease. Neuron 16, 1049–1060 (1996).
Lupski, J.R. et al. DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell 66, 219–232 (1991).
Raeymaekers, P. Duplication in chromosome 17p11.2 in Charcot-Marie-Tooth neuropathy type 1a (CMT 1a). Neuromuscul. Disord. 1, 93–97 (1991).
Pareyson, D. & Marchesi, C. Diagnosis, natural history, and management of Charcot-Marie-Tooth disease. Lancet Neurol. 8, 654–667 (2009).
Fledrich, R., Stassart, R.M. & Sereda, M.W. Murine therapeutic models for Charcot-Marie-Tooth (CMT) disease. Br. Med. Bull. 102, 89–113 (2012).
Patzkó, A. & Shy, M.E. Update on Charcot-Marie-Tooth disease. Curr. Neurol. Neurosci. Rep. 11, 78–88 (2011).
Berciano, J., García, A., Calleja, J. & Combarros, O. Clinico-electrophysiological correlation of extensor digitorum brevis muscle atrophy in children with Charcot-Marie-Tooth disease 1A duplication. Neuromuscul. Disord. 10, 419–424 (2000).
Harding, A.E. & Thomas, P.K. The clinical features of hereditary motor and sensory neuropathy types I and II. Brain 103, 259–280 (1980).
Thomas, P.K. et al. The phenotypic manifestations of chromosome 17p11.2 duplication. Brain 120, 465–478 (1997).
Burns, J., Ryan, M.M. & Ouvrier, R.A. Evolution of foot and ankle manifestations in children with CMT1A. Muscle Nerve 39, 158–166 (2009).
Yiu, E.M., Burns, J., Ryan, M.M. & Ouvrier, R.A. Neurophysiologic abnormalities in children with Charcot-Marie-Tooth disease type 1A. J. Peripher. Nerv. Syst. 13, 236–241 (2008).
Fledrich, R. et al. A rat model of Charcot-Marie-Tooth disease 1A recapitulates disease variability and supplies biomarkers of axonal loss in patients. Brain 135, 72–87 (2012).
Sereda, M.W., Meyer zu Hörste, G., Suter, U., Uzma, N. & Nave, K.-A. Therapeutic administration of progesterone antagonist in a model of Charcot-Marie-Tooth disease (CMT–1A). Nat. Med. 9, 1533–1537 (2003).
Gabreëls-Festen, A.A. et al. Charcot-Marie-Tooth disease type 1A: morphological phenotype of the 17p duplication versus PMP22 point mutations. Acta Neuropathol. 90, 645–649 (1995).
Kobsar, I., Hasenpusch–Theil, K., Wessig, C., Müller, H.W. & Martini, R. Evidence for macrophage-mediated myelin disruption in an animal model for Charcot-Marie-Tooth neuropathy type 1A. J. Neurosci. Res. 81, 857–864 (2005).
Jessen, K.R. & Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 6, 671–682 (2005).
Hanemann, C.O., Gabreëls–Festen, A.A.W.M., Stoll, G. & Müller, H.W. Schwann cell differentiation in Charcot-Marie-Tooth disease type 1A (CMT1A): normal number of myelinating Schwann cells in young CMT1A patients and neural cell adhesion molecule expression in onion bulbs. Acta Neuropathol. 94, 310–315 (1997).
Hanemann, C.O., Gabreëls–Fasten, A.A.W.M., Müller, H.W. & Stoll, G. Low affinity NGF receptor expression in CMT1A nerve biopsies of different disease stages. Brain 119, 1461–1469 (1996).
Adlkofer, K. et al. Hypermyelination and demyelianting peripheral neuropathy in Pmp22-deficient mice. Nat. Genet. 11, 274–280 (1995).
Martini, R., Klein, D. & Groh, J. Similarities between inherited demyelinating neuropathies and Wallerian degeneration: an old repair program may cause myelin and axon perturbation under nonlesion conditions. Am. J. Pathol. 183, 655–660 (2013).
Fischer, S., Weishaupt, A., Troppmair, J. & Martini, R. Increase of MCP-1 (CCL2) in myelin mutant Schwann cells is mediated by Mek-Erk signaling pathway. Glia 56, 836–843 (2008).
Kohl, B., Fischer, S., Groh, J., Wessig, C. & Martini, R. MCP-1/CCL2 modifies axon properties in a PMP22-overexpressing mouse model for Charcot-Marie-Tooth 1A neuropathy. Am. J. Pathol. 176, 1390–1399 (2010).
Napoli, I. et al. A central role for the Erk-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron 73, 729–742 (2012).
Mendoza, M.C., Emrah Er, E. & Blenis, J. The Ras-Erk and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem. Sci. 36, 320–328 (2011).
Nobbio, L. et al. Impairment of PMP22 transgenic Schwann cells differentiation in culture: implications for Charcot-Marie-Tooth type 1A disease. Neurobiol. Dis. 16, 263–273 (2004).
Syed, N. et al. Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. J. Neurosci. 30, 6122–6131 (2010).
Taveggia, C. et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 47, 681–694 (2005).
Birchmeier, C. & Nave, K.-A. Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia 56, 1491–1497 (2008).
Michailov, G.V. et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science 304, 700–703 (2004).
Falls, D.L. Neuregulins and the neuromuscular system: 10 years of answers and questions. J. Neurocytol. 32, 619–647 (2003).
Stassart, R.M. et al. A role for Schwann cell–derived neuregulin-1 in remyelination. Nat. Neurosci. 16, 48–54 (2013).
Mendes–Ferreira, P., De Keulenaer, G.W., Leite–Moreira, A.F. & Brás–Silva, C. Therapeutic potential of neuregulin-1 in cardiovascular disease. Drug Discov. Today 18, 836–842 (2013).
Huxley, C. et al. Correlation between varying levels of PMP22 expression and the degree of demyelination and reduction in nerve conduction velocity in transgenic mice. Hum. Mol. Genet. 7, 449–458 (1998).
Bersell, K., Arab, S., Haring, B. & Kühn, B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 138, 257–270 (2009).
Kato, T. et al. Transient exposure of neonatal mice to neuregulin-1 results in hyperdopaminergic states in adulthood: implication in neurodevelopmental hypothesis for schizophrenia. Mol. Psychiatry 16, 307–320 (2011).
Haney, C. et al. Ultrastructural distribution of PMP22 in Charcot-Marie-Tooth disease type 1A. J. Neuropathol. Exp. Neurol. 55, 290–299 (1996).
Koike, H. et al. Nonmyelinating Schwann cell involvement with well-preserved unmyelinated axons in Charcot-Marie-Tooth disease type 1A. J. Neuropathol. Exp. Neurol. 66, 1027–1036 (2007).
Arthur–Farraj, P.J. et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 75, 633–647 (2012).
D'Antonio, M. et al. Resetting translational homeostasis restores myelination in Charcot-Marie-Tooth disease type 1B mice. J. Exp. Med. 210, 821–838 (2013).
Patzkó, A. et al. Curcumin derivatives promote Schwann cell differentiation and improve neuropathy in R98C CMT1B mice. Brain 135, 3551–3566 (2012).
Meyer zu Hörste, G. et al. Antiprogesterone therapy uncouples axonal loss from demyelination in a transgenic rat model of CMT1A neuropathy. Ann. Neurol. 61, 61–72 (2007).
Brockes, J.P. et al. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 165, 105–118 (1979).
Krishnan, A.V., Lin, C.S.-Y., Park, S.B. & Kiernan, M.C. Axonal ion channels from bench to bedside: a translational neuroscience perspective. Prog. Neurobiol. 89, 288–313 (2009).
Nodera, H. et al. Nerve excitability properties in Charcot-Marie-Tooth disease type 1A. Brain 127, 203–211 (2004).
Boërio, D., Greensmith, L. & Bostock, H. Excitability properties of motor axons in the maturing mouse. J. Peripher. Nerv. Syst. 14, 45–53 (2009).
Acknowledgements
We thank U. Suter (Eidgenössische Technische Hochschule Zurich) for providing Pmp22-deficient mice and C. Huxley (Imperial College School of Medicine, London) for providing Pmp22-transgenic mice. We are grateful to T. Durkaya, C. Maack, A. Fahrenholz, T. Freerck, M. Wehe and A. Wohltmann for technical support. We thank W. Möbius and T. Ruhwedel for help with electron microscopy. This work was supported by grants of the German Research Foundation (DFG) Research Center Molecular Physiology of the Brain, European Commission FP7-201535 (Neuron-Glia Interactions in Nerve Development and Disease) to K.-A.N. M.W.S. and R.F. were supported by the German Ministry of Education and Research (BMBF, FKZ 01ES0812 to M.W.S.). M.W.S. was also supported by the Association Francaise contre les Myopathies (NR 15037) and holds a Heisenberg Professorship granted by the DFG (GZ: SE 1944/1-1). K.-A.N. holds a European Research Council Advanced Investigator Grant.
Author information
Authors and Affiliations
Contributions
R.F. and R.M.S. designed the study, performed experiments and wrote the manuscript. A.K., T.P., L.M.R., T.K., T.A.M.A. and N.K. contributed to experiments. L.H. and D.C. performed threshold electrotonus measurements. C.S. and W.B. contributed to immunohistochemistry and discussions. M.W.S. and K.-A.N. contributed to the manuscript and supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 7484 kb)
Rights and permissions
About this article
Cite this article
Fledrich, R., Stassart, R., Klink, A. et al. Soluble neuregulin-1 modulates disease pathogenesis in rodent models of Charcot-Marie-Tooth disease 1A. Nat Med 20, 1055–1061 (2014). https://doi.org/10.1038/nm.3664
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3664
This article is cited by
-
AAV-mediated editing of PMP22 rescues Charcot-Marie-Tooth disease type 1A features in patient-derived iPS Schwann cells
Communications Medicine (2023)
-
HDAC3 Inhibition Stimulates Myelination in a CMT1A Mouse Model
Molecular Neurobiology (2022)
-
Lessons from Injury: How Nerve Injury Studies Reveal Basic Biological Mechanisms and Therapeutic Opportunities for Peripheral Nerve Diseases
Neurotherapeutics (2021)
-
Mechanisms and Treatments in Demyelinating CMT
Neurotherapeutics (2021)
-
CMTM6 expressed on the adaxonal Schwann cell surface restricts axonal diameters in peripheral nerves
Nature Communications (2020)