Abstract
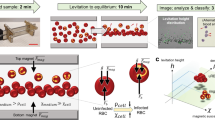
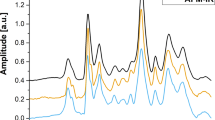
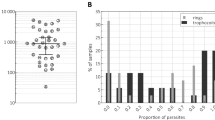
We report a new technique for sensitive, quantitative and rapid detection of Plasmodium spp.–infected red blood cells (RBCs) by means of magnetic resonance relaxometry (MRR). During the intraerythrocytic cycle, malaria parasites metabolize large amounts of cellular hemoglobin and convert it into hemozoin crystallites. We exploit the relatively large paramagnetic susceptibility of these hemozoin particles, which induce substantial changes in the transverse relaxation rate of proton nuclear magnetic resonance of RBCs, to infer the 'parasite load' in blood. Using an inexpensive benchtop 0.5-Tesla MRR system, we show that with minimal sample preparatory steps and without any chemical or immunolabeling, a parasitemia level of fewer than ten parasites per microliter in a volume below 10 μl of whole blood is detected in a few minutes. We demonstrate this method both for cultured Plasmodium falciparum parasites and in vivo with Plasmodium berghei–infected mice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
WHO. World Malaria Report 2013 (World Health Organization, Geneva, 2013).
Warhurst, D.C. & Williams, J. ACP Broadsheet no 148. July 1996. Laboratory diagnosis of malaria. J. Clin. Pathol. 49, 533–538 (1996).
Amexo, M., Tolhurst, R., Barnish, G. & Bates, I. Malaria misdiagnosis: effects on the poor and vulnerable. Lancet 364, 1896–1898 (2004).
Caramello, P., Lucchini, A., Savoia, D. & Gioannini, P. Rapid diagnosis of malaria by use of fluorescent probes. Diagn. Microbiol. Infect. Dis. 17, 293–297 (1993).
Moody, A. Rapid diagnostic tests for malaria parasites. Clin. Microbiol. Rev. 15, 66–78 (2002).
Makler, M.T., Ries, L., Ries, J., Horton, R. & Hinrichs, D. Detection of Plasmodium falciparum infection with the fluorescent dye, benzothiocarboxypurine. Am. J. Trop. Med. Hyg. 44, 11–16 (1991).
Lee, H., Sun, E., Ham, D. & Weissleder, R. Chip–NMR biosensor for detection and molecular analysis of cells. Nat. Med. 14, 869–874 (2008).
Lee, H., Yoon, T.-J., Figueiredo, J.-L., Swirski, F.K. & Weissleder, R. Rapid detection and profiling of cancer cells in fine-needle aspirates. Proc. Natl. Acad. Sci. USA 106, 12459–12464 (2009).
Peng, W.K. & Han, J. Biosensor, palm-sized device and method based on Magnetic Resonance Relaxometry. WO Patent WO2012118442 (2012).
Peng, W.K., Chen, L. & Han, J. Development of miniaturized, portable magnetic resonance relaxometry system for point-of-care medical diagnosis. Rev. Sci. Instrum. 83, 095115 (2012).
Vo, N.N. et al. Highly integrated, low cost, palm-top sized magnetic resonance relaxometry system for rapid blood screening. IFMBE Proc. 43, 558–561 (2014).
Sullivan, D. Jr. Hemozoin: a biocrystal synthesized during the degradation of hemoglobin. Biopolym. Online 10.1002/3527600035.bpol9007 (15 January 2005).
Sullivan, D.J., Gluzman, I.Y. & Goldberg, D.E. Plasmodium hemozoin formation mediated by histidine-rich proteins. Science 271, 219–222 (1996).
Hackett, S., Hamzah, J., Davis, T.M. & St Pierre, T.G. Magnetic susceptibility of iron in malaria-infected red blood cells. Biochim. Biophys. Acta 1792, 93–99 (2009).
Bradley, D. Malaria. in Oxford Textbook of Medicine (ed. Weatherall, D.J.) 835–863 (Oxford Medical Publishers, 2003).
Meyer, M.E., Yu, O., Eclancher, B., Grucker, D. & Chambron, J. NMR relaxation rates and blood oxygenation level. Magn. Reson. Med. 34, 234–241 (1995).
Thulborn, K.R., Waterton, J.C., Matthews, P.M. & Radda, G.K. Oxygenation dependence of the transverse relaxation-time of water protons in whole-blood at high-field. Biochim. Biophys. Acta 714, 265–270 (1982).
Eccles, C. Low field NMR methods and applications. in Encyclopedia of Spectroscopy and Spectrometry (ed. Lindon, J.C.) 1357–1371 (Academic Press, Oxford, 2010).
Lambros, C. & Vanderberg, J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65, 418–420 (1979).
Ribaut, C. et al. Concentration and purification by magnetic separation of the erythrocytic stages of all human Plasmodium species. Malar. J. 7, 45 (2008).
Silamut, K. et al. A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am. J. Pathol. 155, 395–410 (1999).
Goodman, A.L. et al. The utility of Plasmodium berghei as a rodent model for anti-merozoite malaria vaccine assessment. Sci. Rep. 3, 1706 (2013).
Yañez, D.M., Manning, D.D., Cooley, A.J., Weidanz, W.P. & Van Der Heyde, H. Participation of lymphocyte subpopulations in the pathogenesis of experimental murine cerebral malaria. J. Immunol. 157, 1620–1624 (1996).
Webb, G.A. Radiofrequency microcoils in magnetic resonance. Prog. Nucl. Magn. Reson. Spectrosc. 31, 1–42 (1997).
Karl, S., Gutiérrez, L., House, M.J., Davis, T.M. & Pierre, T.G.S. Nuclear magnetic resonance: a tool for malaria diagnosis? Am. J. Trop. Med. Hyg. 85, 815–817 (2011).
Takeda, K. A highly integrated FPGA-based nuclear magnetic resonance spectrometer. Rev. Sci. Instrum. 78, 033103 (2007).
Saul, A., Myler, P., Elliott, T. & Kidson, C. Purification of mature schizonts of Plasmodium falciparum on colloidal silica gradients. Bull. World Health Organ. 60, 755–759 (1982).
Tosta, C.E., Sedegah, M., Henderson, D. & Wedderburn, N. Plasmodium yoelii and Plasmodium berghei: Isolation of infected erythrocytes from blood by colloidal silica gradient centrifugation. Exp. Parasitol. 50, 7–15 (1980).
Rüssmann, L., Jung, A. & Heidrich, H.-G. The use of Percoll gradients, elutriator rotor elution, and mithramycin staining for the isolation and identification of intraerythrocytic stages of Plasmodium berghei. Z. Parasitenkd. 66, 273–280 (1982).
Eling, W. Ficoll fractionation for the separation of parasitized erythrocytes from malaria infected blood. Bull. World Health Organ. 55, 105–114 (1977).
Crewe, W. Basic Malaria Microscopy 2nd edn. 219 (World Health Organization, 2010).
Acknowledgements
This work is supported by the National Research Foundation Singapore under its SMART Centre, BioSystems and Micromechanics IRG and Infectious Disease IRG. W.K.P. acknowledges support from the SMART Postdoctoral Research Fellows Programme and SMART Ignition Grant (ING 11025-BIO(IGN)) and K.R. Roy for culturing and preparing the sample parasites. T.F.K. and C.S.N. acknowledge financial support from the Singapore-MIT Alliance (SMA) Graduate Fellowship.
Author information
Authors and Affiliations
Contributions
W.K.P. and J.H. conceived the original idea and designed the study. W.K.P. built the whole experimental setup, designed the protocol and performed MRR measurements of P. falciparum and P. berghei. L.C. assisted in radiofrequency probe design. C.S.N., W.K.P., A.A.S.B. and L.C. were involved in preparing P. falciparum parasite samples. T.F.K., C.S.N., W.K.P. and Y.H. worked on the in vivo mice studies (day-to-day measurements). C.S.N., W.K.P., L.C. and T.F.K. contributed equally to the blinded mouse studies. P.R.P., N.-T.N. and J.H. supervised the mouse work. W.K.P., P.R.P., J.H., T.F.K. and C.S.N. wrote the paper, and all the authors checked through the manuscript and analyzed the data.
Corresponding authors
Ethics declarations
Competing interests
W.K.P. and J.H. have filed patents with the World Intellectual Property Office on the technique discussed here: a technology patent describing the strategies for miniaturization of the devices and an application patent describing its usage as a malaria screening tool (ref. 9).
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–5, Supplementary Figures 1–10 Supplementary Results and Supplementary Discussion (PDF 3343 kb)
Rights and permissions
About this article
Cite this article
Peng, W., Kong, T., Ng, C. et al. Micromagnetic resonance relaxometry for rapid label-free malaria diagnosis. Nat Med 20, 1069–1073 (2014). https://doi.org/10.1038/nm.3622
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3622
This article is cited by
-
Novel time-domain NMR-based traits for rapid, label-free Olive oils profiling
npj Science of Food (2022)
-
Magneto-optical diagnosis of symptomatic malaria in Papua New Guinea
Nature Communications (2021)
-
Rapid phenotyping towards personalized malaria medicine
Malaria Journal (2020)
-
Machine learning assistive rapid, label-free molecular phenotyping of blood with two-dimensional NMR correlational spectroscopy
Communications Biology (2020)
-
Molecular phenotyping of oxidative stress in diabetes mellitus with point-of-care NMR system
npj Aging and Mechanisms of Disease (2020)