Abstract
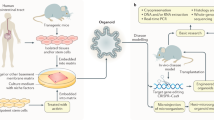
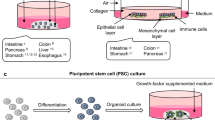
The application of primary organoid cultures containing epithelial and mesenchymal elements to cancer modeling holds promise for combining the accurate multilineage differentiation and physiology of in vivo systems with the facile in vitro manipulation of transformed cell lines. Here we used a single air-liquid interface culture method without modification to engineer oncogenic mutations into primary epithelial and mesenchymal organoids from mouse colon, stomach and pancreas. Pancreatic and gastric organoids exhibited dysplasia as a result of expression of Kras carrying the G12D mutation (KrasG12D), p53 loss or both and readily generated adenocarcinoma after in vivo transplantation. In contrast, primary colon organoids required combinatorial Apc, p53, KrasG12D and Smad4 mutations for progressive transformation to invasive adenocarcinoma-like histology in vitro and tumorigenicity in vivo, recapitulating multi-hit models of colorectal cancer (CRC), as compared to the more promiscuous transformation of small intestinal organoids. Colon organoid culture functionally validated the microRNA miR-483 as a dominant driver oncogene at the IGF2 (insulin-like growth factor-2) 11p15.5 CRC amplicon, inducing dysplasia in vitro and tumorigenicity in vivo. These studies demonstrate the general utility of a highly tractable primary organoid system for cancer modeling and driver oncogene validation in diverse gastrointestinal tissues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hynds, R.E. & Giangreco, A. Concise review: the relevance of human stem cell–derived organoid models for epithelial translational medicine. Stem Cells 31, 417–422 (2013).
Sato, T. & Clevers, H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 340, 1190–1194 (2013).
Stelzner, M. et al. A nomenclature for intestinal in vitro cultures. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G1359–G1363 (2012).
Barker, N. et al. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25–36 (2010).
Pylayeva-Gupta, Y., Lee, K.E. & Bar-Sagi, D. Microdissection and culture of murine pancreatic ductal epithelial cells. Methods Mol. Biol. 980, 267–279 (2013).
Jin, L. et al. Colony-forming cells in the adult mouse pancreas are expandable in Matrigel and form endocrine/acinar colonies in laminin hydrogel. Proc. Natl. Acad. Sci. USA 110, 3907–3912 (2013).
Smukler, S.R. et al. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell 8, 281–293 (2011).
Huch, M. et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 32, 2708–2721 (2013).
Ootani, A. et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 15, 701–706 (2009).
Katano, T. et al. Establishment of a long-term three-dimensional primary culture of mouse glandular stomach epithelial cells within the stem cell niche. Biochem. Biophys. Res. Commun. 432, 558–563 (2013).
Ghajar, C.M. & Bissell, M.J. Tumor engineering: the other face of tissue engineering. Tissue Eng. Part A 16, 2153–2156 (2010).
Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337 (2012).
Wescott, M.P. et al. Pancreatic ductal morphogenesis and the Pdx1 homeodomain transcription factor. Mol. Biol. Cell 20, 4838–4844 (2009).
Rovira, M. et al. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc. Natl. Acad. Sci. USA 107, 75–80 (2010).
Hingorani, S.R. et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7, 469–483 (2005).
Fearon, E.R. & Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 61, 759–767 (1990).
Haigis, K.M. et al. Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat. Genet. 40, 600–608 (2008).
Sansom, O.J. et al. Loss of Apc allows phenotypic manifestation of the transforming properties of an endogenous K-ras oncogene in vivo. Proc. Natl. Acad. Sci. USA 103, 14122–14127 (2006).
el Marjou, F. et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39, 186–193 (2004).
Shibata, H. et al. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science 278, 120–123 (1997).
Hung, K.E. et al. Development of a mouse model for sporadic and metastatic colon tumors and its use in assessing drug treatment. Proc. Natl. Acad. Sci. USA 107, 1565–1570 (2010).
Taketo, M.M. & Edelmann, W. Mouse models of colon cancer. Gastroenterology 136, 780–798 (2009).
Tanaka, T., Soriano, M.A. & Grusby, M.J. SLIM is a nuclear ubiquitin E3 ligase that negatively regulates STAT signaling. Immunity 22, 729–736 (2005).
Attali, M. et al. Control of beta-cell differentiation by the pancreatic mesenchyme. Diabetes 56, 1248–1258 (2007).
Duvillié, B., Heinis, M. & Stetsyuk, V. In vivo and in vitro techniques to study pancreas development and islet cell function. Endocr. Dev. 12, 46–54 (2007).
Lee, K.E. & Bar-Sagi, D. Oncogenic KRas suppresses inflammation-associated senescence of pancreatic ductal cells. Cancer Cell 18, 448–458 (2010).
Onuma, K. et al. Genetic reconstitution of tumorigenesis in primary intestinal cells. Proc. Natl. Acad. Sci. USA 110, 11127–11132 (2013).
Leung, C.T. & Brugge, J.S. Outgrowth of single oncogene-expressing cells from suppressive epithelial environments. Nature 482, 410–413 (2012).
Kim, J. et al. An iPSC line from human pancreatic ductal adenocarcinoma undergoes early to invasive stages of pancreatic cancer progression. Cell Reports 3, 2088–2099 (2013).
Vogelstein, B. et al. Cancer genome landscapes. Science 339, 1546–1558 (2013).
March, H.N. et al. Insertional mutagenesis identifies multiple networks of cooperating genes driving intestinal tumorigenesis. Nat. Genet. 43, 1202–1209 (2011).
Starr, T.K. et al. A transposon-based genetic screen in mice identifies genes altered in colorectal cancer. Science 323, 1747–1750 (2009).
Barretina, J. et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483, 603–607 (2012).
Firestein, R. et al. CDK8 is a colorectal cancer oncogene that regulates β-catenin activity. Nature 455, 547–551 (2008).
Veronese, A. et al. Oncogenic role of miR-483–3p at the IGF2/483 locus. Cancer Res. 70, 3140–3149 (2010).
Harper, J. et al. Soluble IGF2 receptor rescues ApcMin/+ intestinal adenoma progression induced by Igf2 loss of imprinting. Cancer Res. 66, 1940–1948 (2006).
Timp, W., Levchenko, A. & Feinberg, A.P. A new link between epigenetic progenitor lesions in cancer and the dynamics of signal transduction. Cell Cycle 8, 383–390 (2009).
Hassan, A.B. & Howell, J.A. Insulin-like growth factor II supply modifies growth of intestinal adenoma in ApcMin/+ mice. Cancer Res. 60, 1070–1076 (2000).
Qu, Z. et al. DNA methylation–dependent repression of PDZ-LIM domain–containing protein 2 in colon cancer and its role as a potential therapeutic target. Cancer Res. 70, 1766–1772 (2010).
Spence, J.R. et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109 (2011).
Sasai, K. et al. Oncogene-mediated human lung epithelial cell transformation produces adenocarcinoma phenotypes in vivo. Cancer Res. 71, 2541–2549 (2011).
Dickins, R.A. et al. Probing tumor phenotypes using stable and regulated synthetic microRNA precursors. Nat. Genet. 37, 1289–1295 (2005).
Griffiths-Jones, S., Saini, H.K., van Dongen, S. & Enright, A.J. miRBase: tools for microRNA genomics. Nucleic Acids Res. 36, D154–D158 (2008).
Acknowledgements
We are grateful to members of the Kuo laboratory, M. Amieva, R. Honaker, T. Jacks and C. Chartier for helpful discussion, P. Chu and S. Michie for assistance with histology, S. Lowe (Memorial Sloan Kettering Cancer Center) for LMP-based retroviruses, B. Williams (Van Andel Research Institute) for mouse strains, R. Mulligan and J.-S. Lee (Harvard University) and J. Chen (Stanford University) for lentiviral and retroviral reagents. X.L. was supported by the Dean's Fellowship at Stanford University, A.O. was supported by a Physician-Scientist Fellowship from the California Institute for Regenerative Medicine, J.T.N. was supported by a Postdoctoral Fellowship (124574-PF-13-296-01-TBG) from the American Cancer Society, C.W.-M.C. was supported by the Croucher Foundation, and T.Y. was supported by the Royal College of Surgeons. L.N. was supported by an American Society for Clinical Oncology Young Investigator Award and US National Institutes of Health grant K08CA166512. C.-Z.C. was supported by grants from the US National Institutes of Health (1R01AI073724 and Pioneer Award DP1CA174421) and the W.M. Keck Foundation. We thank the generous support from the Innovate Foundation and the Krishnan-Shah Family Foundation to C.J.K. This work was also supported by US National Institutes of Health grants K08DK078033 and U01CA084301 to K.E.H.; US National Cancer Institute Integrative Cancer Biology Program grant U01CA151920, US National Cancer Institute Cancer Target Discovery and Development grant 1U01CA168424 and a Fidelity Foundation grant to C.J.K. and H.P.J.; and the US National Institutes of Health Transformative R01DK085720 and US National Institute of Diabetes and Digestive and Kidney Diseases Intestinal Stem Cell Consortium grant U01DK085527 to C.J.K.
Author information
Authors and Affiliations
Contributions
X.L., L.N., A.O., D.C.C., M.A.C., P.G.R. and J.T.N. designed and executed organoid experiments. R.K.P. performed blinded histologic interpretation. O.G. and X.G. performed bioinformatic analyses. C.W.-M.C., T.Y., J.Y. and J.W. performed organoid experiments and produced viruses. S.R., L.D.A. and K.E.H. provided reagents. K.E.H., C.-Z.C., S.K.P., H.P.J. and C.J.K. designed experiments. X.L., L.N., D.C.C. and C.J.K. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
C.J.K., L.N. and A.O. have filed patents with the United States Patent and Trademark Office to cover the use of this organoid technique for cancer modeling.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 and Supplementary Discussion (PDF 13480 kb)
Rights and permissions
About this article
Cite this article
Li, X., Nadauld, L., Ootani, A. et al. Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nat Med 20, 769–777 (2014). https://doi.org/10.1038/nm.3585
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3585
This article is cited by
-
Dual targeting of MAPK and PI3K pathways unlocks redifferentiation of Braf-mutated thyroid cancer organoids
Oncogene (2024)
-
Promising preclinical patient-derived organoid (PDO) and xenograft (PDX) models in upper gastrointestinal cancers: progress and challenges
BMC Cancer (2023)
-
Breast cancer organoids and their applications for precision cancer immunotherapy
World Journal of Surgical Oncology (2023)
-
A new scaffold-free tumoroid model provides a robust preclinical tool to investigate invasion and drug response in Renal Cell Carcinoma
Cell Death & Disease (2023)
-
Peristalsis-Associated Mechanotransduction Drives Malignant Progression of Colorectal Cancer
Cellular and Molecular Bioengineering (2023)