Abstract
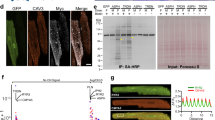
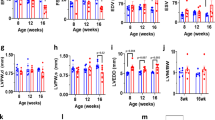
Cardiomyocyte T tubules are important for regulating ion flux. Bridging integrator 1 (BIN1) is a T-tubule protein associated with calcium channel trafficking that is downregulated in failing hearts. Here we find that cardiac T tubules normally contain dense protective inner membrane folds that are formed by a cardiac isoform of BIN1. In mice with cardiac Bin1 deletion, T-tubule folding is decreased, which does not change overall cardiomyocyte morphology but leads to free diffusion of local extracellular calcium and potassium ions, prolonging action-potential duration and increasing susceptibility to ventricular arrhythmias. We also found that T-tubule inner folds are rescued by expression of the BIN1 isoform BIN1+13+17, which promotes N-WASP–dependent actin polymerization to stabilize the T-tubule membrane at cardiac Z discs. BIN1+13+17 recruits actin to fold the T-tubule membrane, creating a 'fuzzy space' that protectively restricts ion flux. When the amount of the BIN1+13+17 isoform is decreased, as occurs in acquired cardiomyopathy, T-tubule morphology is altered, and arrhythmia can result.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Soeller, C. & Cannell, M.B. Examination of the transverse tubular system in living cardiac rat myocytes by 2-photon microscopy and digital image-processing techniques. Circ. Res. 84, 266–275 (1999).
Brette, F. & Orchard, C. T-tubule function in mammalian cardiac myocytes. Circ. Res. 92, 1182–1192 (2003).
Cheng, H., Lederer, W.J. & Cannell, M.B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science 262, 740–744 (1993).
Bers, D.M. Cardiac excitation-contraction coupling. Nature 415, 198–205 (2002).
Pásek, M., Simurda, J. & Christe, G. The functional role of cardiac T-tubules explored in a model of rat ventricular myocytes. Philos. Trans. A. Math. Phys. Eng. Sci. 364, 1187–1206 (2006).
Lederer, W.J., Niggli, E. & Hadley, R.W. Sodium-calcium exchange in excitable cells: fuzzy space. Science 248, 283 (1990).
Shepherd, N. & McDonough, H.B. Ionic diffusion in transverse tubules of cardiac ventricular myocytes. Am. J. Physiol. 275, H852–H860 (1998).
Swift, F., Stromme, T.A., Amundsen, B., Sejersted, O.M. & Sjaastad, I. Slow diffusion of K+ in the T tubules of rat cardiomyocytes. J. Appl. Physiol. 101, 1170–1176 (2006).
Parfenov, A.S., Salnikov, V., Lederer, W.J. & Lukyanenko, V. Aqueous diffusion pathways as a part of the ventricular cell ultrastructure. Biophys. J. 90, 1107–1119 (2006).
Pásek, M., Simurda, J. & Orchard, C.H. Role of T-tubules in the control of trans-sarcolemmal ion flux and intracellular Ca2+ in a model of the rat cardiac ventricular myocyte. Eur. Biophys. J. 41, 491–503 (2012).
Pásek, M., Simurda, J., Orchard, C.H. & Christe, G. A model of the guinea-pig ventricular cardiac myocyte incorporating a transverse-axial tubular system. Prog. Biophys. Mol. Biol. 96, 258–280 (2008).
Lyon, A.R. et al. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc. Natl. Acad. Sci. USA 106, 6854–6859 (2009).
Wei, S. et al. T-tubule remodeling during transition from hypertrophy to heart failure. Circ. Res. 107, 520–531 (2010).
Wagner, E. et al. Stimulated emission depletion live-cell super-resolution imaging shows proliferative remodeling of T-tubule membrane structures after myocardial infarction. Circ. Res. 111, 402–414 (2012).
Aiba, T. & Tomaselli, G.F. Electrical remodeling in the failing heart. Curr. Opin. Cardiol. 25, 29–36 (2010).
Sipido, K.R. Calcium overload, spontaneous calcium release, and ventricular arrhythmias. Heart Rhythm 3, 977–979 (2006).
Lee, E. et al. Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science 297, 1193–1196 (2002).
Hong, T.T. et al. BIN1 localizes the L-type calcium channel to cardiac T-tubules. PLoS Biol. 8, e1000312 (2010).
Hong, T.T. et al. BIN1 is reduced and Cav1.2 trafficking is impaired in human failing cardiomyocytes. Heart Rhythm 9, 812–820 (2012).
Lyon, A.R. et al. Plasticity of surface structures and β2-adrenergic receptor localization in failing ventricular cardiomyocytes during recovery from heart failure. Circ Heart Fail 5, 357–365 (2012).
Böhm, J. et al. Case report of intrafamilial variability in autosomal recessive centronuclear myopathy associated to a novel BIN1 stop mutation. Orphanet J. Rare Dis. 5, 35 (2010).
Muller, A.J. et al. Targeted disruption of the murine Bin1/Amphiphysin II gene does not disable endocytosis but results in embryonic cardiomyopathy with aberrant myofibril formation. Mol. Cell. Biol. 23, 4295–4306 (2003).
Agah, R. et al. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J. Clin. Invest. 100, 169–179 (1997).
Chang, M. Y. et al. Bin1 ablation in mammary gland delays tissue remodeling and drives cancer progression. Cancer Res. 67, 100–107 (2007).
Goonasekera, S.A. et al. Decreased cardiac L-type Ca2+ channel activity induces hypertrophy and heart failure in mice. J. Clin. Invest. 122, 280–290 (2012).
Kawai, M., Hussain, M. & Orchard, C.H. Excitation-contraction coupling in rat ventricular myocytes after formamide-induced detubulation. Am. J. Physiol. 277, H603–H609 (1999).
Hepler, P.K. Membranes in the mitotic apparatus of barley cells. J. Cell Biol. 86, 490–499 (1980).
Franzini-Armstrong, C. Simultaneous maturation of transverse tubules and sarcoplasmic reticulum during muscle differentiation in the mouse. Dev. Biol. 146, 353–363 (1991).
Komukai, K., Brette, F., Yamanushi, T.T. & Orchard, C.H. K+ current distribution in rat sub-epicardial ventricular myocytes. Pflugers Arch. 444, 532–538 (2002).
Pásek, M. et al. Quantification of T-tubule area and protein distribution in rat cardiac ventricular myocytes. Prog. Biophys. Mol. Biol. 96, 244–257 (2008).
Shaw, R.M. & Rudy, Y. Electrophysiologic effects of acute myocardial ischemia. A mechanistic investigation of action potential conduction and conduction failure. Circ. Res. 80, 124–138 (1997).
Mathur, N. et al. Sudden infant death syndrome in mice with an inherited mutation in RyR2. Circ. Arrhythm. Electrophysiol. 2, 677–685 (2009).
Kannankeril, P.J. et al. Mice with the R176Q cardiac ryanodine receptor mutation exhibit catecholamine-induced ventricular tachycardia and cardiomyopathy. Proc. Natl. Acad. Sci. USA 103, 12179–12184 (2006).
Marban, E., Robinson, S.W. & Wier, W.G. Mechanisms of arrhythmogenic delayed and early afterdepolarizations in ferret ventricular muscle. J. Clin. Invest. 78, 1185–1192 (1986).
Tian, Q. et al. Functional and morphological preservation of adult ventricular myocytes in culture by sub-micromolar cytochalasin D supplement. J. Mol. Cell. Cardiol. 52, 113–124 (2012).
Yamada, H. et al. Dynamic interaction of amphiphysin with N-WASP regulates actin assembly. J. Biol. Chem. 284, 34244–34256 (2009).
Gaur, N., Rudy, Y. & Hool, L. Contributions of ion channel currents to ventricular action potential changes and induction of early afterdepolarizations during acute hypoxia. Circ. Res. 105, 1196–1203 (2009).
Kim, J.M., Bursac, N. & Henriquez, C.S. A computer model of engineered cardiac monolayers. Biophys. J. 98, 1762–1771 (2010).
Gez, L.S., Hagalili, Y., Shainberg, A. & Atlas, D. Voltage-driven Ca2+ binding at the L-type Ca2+ channel triggers cardiac excitation-contraction coupling prior to Ca2+ influx. Biochemistry 51, 9658–9666 (2012).
Taylor, M.J., Perrais, D. & Merrifield, C.J. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol. 9, e1000604 (2011).
Butler, M.H. et al. Amphiphysin II (SH3P9; BIN1), a member of the amphiphysin/Rvs family, is concentrated in the cortical cytomatrix of axon initial segments and nodes of ranvier in brain and around T tubules in skeletal muscle. J. Cell Biol. 137, 1355–1367 (1997).
Böhm, J. et al. Altered splicing of the BIN1 muscle-specific exon in humans and dogs with highly progressive centronuclear myopathy. PLoS Genet. 9, e1003430 (2013).
Ge, K. et al. Mechanism for elimination of a tumor suppressor: aberrant splicing of a brain-specific exon causes loss of function of Bin1 in melanoma. Proc. Natl. Acad. Sci. USA 96, 9689–9694 (1999).
Sakamuro, D., Elliott, K.J., Wechsler-Reya, R. & Prendergast, G.C. BIN1 is a novel MYC-interacting protein with features of a tumour suppressor. Nat. Genet. 14, 69–77 (1996).
Elliott, K. et al. Bin1 functionally interacts with Myc and inhibits cell proliferation via multiple mechanisms. Oncogene 18, 3564–3573 (1999).
Kashef, F. et al. Ankyrin-B protein in heart failure: identification of a new component of metazoan cardioprotection. J. Biol. Chem. 287, 30268–30281 (2012).
Beuckelmann, D.J., Nabauer, M. & Erdmann, E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ. Res. 73, 379–385 (1993).
Smyth, J.W. et al. Actin cytoskeleton rest stops regulate anterograde traffic of connexin 43 vesicles to the plasma membrane. Circ. Res. 110, 978–989 (2012).
O'Connell, T.D., Rodrigo, M.C. & Simpson, P.C. Isolation and culture of adult mouse cardiac myocytes. Methods Mol. Biol. 357, 271–296 (2007).
Hattori, F. et al. Nongenetic method for purifying stem cell–derived cardiomyocytes. Nat. Methods 7, 61–66 (2010).
Leach, R.N., Desai, J.C. & Orchard, C.H. Effect of cytoskeleton disruptors on L-type Ca channel distribution in rat ventricular myocytes. Cell Calcium 38, 515–526 (2005).
Novak, P. et al. Nanoscale live-cell imaging using hopping probe ion conductance microscopy. Nat. Methods 6, 279–281 (2009).
Kremer, J.R., Mastronarde, D.N. & McIntosh, J.R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71–76 (1996).
Mastronarde, D.N. Dual-axis tomography: an approach with alignment methods that preserve resolution. J. Struct. Biol. 120, 343–352 (1997).
Shorten, P.R. & Soboleva, T.K. Anomalous ion diffusion within skeletal muscle transverse tubule networks. Theor. Biol. Med. Model. 4, 18 (2007).
Kits, K.S., de Vlieger, T.A., Kooi, B.W. & Mansvelder, H.D. Diffusion barriers limit the effect of mobile calcium buffers on exocytosis of large dense cored vesicles. Biophys. J. 76, 1693–1705 (1999).
Press, W. Numerical Recipes: The Art of Scientific Computing (Cambridge University Press, 2007).
Higham, N.J. Optimization by direct search in matrix computations. SIAM J. Matrix Anal. Appl. 14, 317–333 (1993).
Nguyen, D.T., Ding, C., Wilson, E., Marcus, G.M. & Olgin, J.E. Pirfenidone mitigates left ventricular fibrosis and dysfunction after myocardial infarction and reduces arrhythmias. Heart Rhythm 7, 1438–1445 (2010).
Salama, G., Kanai, A. & Efimov, I.R. Subthreshold stimulation of Purkinje fibers interrupts ventricular tachycardia in intact hearts. Experimental study with voltage-sensitive dyes and imaging techniques. Circ. Res. 74, 604–619 (1994).
Acknowledgements
This work was supported by US National Institutes of Health grants R01 HL094414 (R.M.S.), R37 MH065334 (L.Y.J.), WT090594 (J.G.), R21 GM100224 (M.G.), T32 HL116273 (S.-S.Z.) and K99/R00 HL109075 (T.H.) and by the American Heart Association (S.-S.Z. and R.M.S.). L.Y.J. is a Howard Hughes Medical Institute investigator. We thank E. Cingolani for helpful advice on designing the in vivo pacing protocol, D. Laury-Kleintop and G.C. Prendergast from the Lankenau Institute for Medical Research for Bin1-loxP mice, J. Mulholland and J.J. Perrino for TEM imaging at the Electron Microscopy Core of the Cell Sciences Imaging Facility at the Stanford University Medical Center, J. Smyth and R. Wirka for helpful discussions and T.S. Fong and T. Hitzeman for technical assistance.
Author information
Authors and Affiliations
Contributions
All authors contributed to study design, analysis of the data and writing of the paper. T.H. was involved in the design and performance of all key experiments. H.Y. did the patch-clamp experiments. S.-S.Z. prepared mouse crossing, adenovirus and Bin1 cloning experiments. H.C.C. and B.S. did the in vivo arrhythmia experiments. M.K. performed the actin polymerization assay. A.B. did the scanning ion-conductance microscopy imaging and analysis of T-tubule topology. H.Z. did the optical mapping studies. M.G. did the mathematical modeling.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Table 1 (PDF 3389 kb)
Rights and permissions
About this article
Cite this article
Hong, T., Yang, H., Zhang, SS. et al. Cardiac BIN1 folds T-tubule membrane, controlling ion flux and limiting arrhythmia. Nat Med 20, 624–632 (2014). https://doi.org/10.1038/nm.3543
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3543
This article is cited by
-
BIN1 in cancer: biomarker and therapeutic target
Journal of Cancer Research and Clinical Oncology (2023)
-
Membrane remodelling triggers maturation of excitation–contraction coupling in 3D-shaped human-induced pluripotent stem cell-derived cardiomyocytes
Basic Research in Cardiology (2023)
-
CMYA5 establishes cardiac dyad architecture and positioning
Nature Communications (2022)
-
Tomatidine-stimulated maturation of human embryonic stem cell-derived cardiomyocytes for modeling mitochondrial dysfunction
Experimental & Molecular Medicine (2022)
-
The neuronal-specific isoform of BIN1 regulates β-secretase cleavage of APP and Aβ generation in a RIN3-dependent manner
Scientific Reports (2022)