Abstract
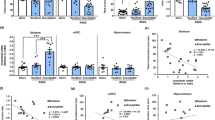
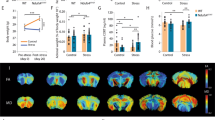
Major depressive disorder (MDD) affects up to 17% of the population, causing profound personal suffering and economic loss1. Clinical and preclinical studies have revealed that prolonged stress and MDD are associated with neuronal atrophy of cortical and limbic brain regions2,3,4,5,6,7,8,9, but the molecular mechanisms underlying these morphological alterations have not yet been identified. Here, we show that stress increases levels of REDD1 (regulated in development and DNA damage responses-1), an inhibitor of mTORC1 (mammalian target of rapamycin complex-1; ref. 10), in rat prefrontal cortex (PFC). This is concurrent with a decrease in phosphorylation of signaling targets of mTORC1, which is implicated in protein synthesis–dependent synaptic plasticity. We also found that REDD1 levels are increased in the postmortem PFC of human subjects with MDD relative to matched controls. Mutant mice with a deletion of the gene encoding REDD1 are resilient to the behavioral, synaptic and mTORC1 signaling deficits caused by chronic unpredictable stress, whereas viral-mediated overexpression of REDD1 in rat PFC is sufficient to cause anxiety- and depressive-like behaviors and neuronal atrophy. Taken together, these postmortem and preclinical findings identify REDD1 as a critical mediator of the atrophy of neurons and depressive behavior caused by chronic stress exposure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kessler, R.C., Chiu, W.T., Demler, O., Merikangas, K.R. & Walters, E.E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 617–627 (2005).
Drevets, W.C. et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386, 824–827 (1997).
Rajkowska, G. et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol. Psychiatry 45, 1085–1098 (1999).
Kang, H.J. et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat. Med. 18, 1413–1417 (2012).
Radley, J.J. et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb. Cortex 16, 313–320 (2006).
Radley, J.J. et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience 125, 1–6 (2004).
Willner, P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52, 90–110 (2005).
Izquierdo, A., Wellman, C.L. & Holmes, A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J. Neurosci. 26, 5733–5738 (2006).
Shansky, R.M., Hamo, C., Hof, P.R., McEwen, B.S. & Morrison, J.H. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb. Cortex 19, 2479–2484 (2009).
Corradetti, M.N. The stress-inducted proteins RTP801 and RTP801L are negative regulators of the mammalian target of rapamycin pathway. J. Biol. Chem. 280, 9769–9772 (2005).
Li, N. et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry 69, 754–761 (2011).
Banasr, M. et al. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol. Psychiatry 62, 496–504 (2007).
Jernigan, C.S. et al. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 1774–1779 (2011).
Li, N. et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329, 959–964 (2010).
Wang, H., Kubica, N., Ellisen, L.W., Jefferson, L.S. & Kimball, S.R. Dexamethasone represses signaling through the mammalian target of rapamycin in muscle cells by enhancing expression of REDD1. J. Biol. Chem. 281, 39128–39134 (2006).
Polman, J.A. et al. Glucocorticoids modulate the mTOR pathway in the hippocampus: differential effects depending on stress history. Endocrinology 153, 4317–4327 (2012).
Boersma, G., Benthem, L., van Dijk, G., Steimer, T.J. & Scheurink, A.J. Pharmacological treatment of hyperinsulineamia in rats depends on coping style. Eur. J. Pharmacol. 654, 122–127 (2011).
Stetler, C. & Miller, G.E. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom. Med. 73, 114–126 (2011).
Feng, Q. et al. The stress-response gene redd1 regulates dorsoventral patterning by antagonizing Wnt/β-catenin activity in zebrafish. PLoS ONE 7, e52674 (2012).
Shoshani, T. et al. Identification of a novel hypoxia-inducible factor 1–responsive gene, RTP801, involved in apoptosis. Mol. Cell. Biol. 22, 2283–2293 (2002).
Liu, R.J. & Aghajanian, G.K. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc. Natl. Acad. Sci. USA 105, 359–364 (2008).
Duman, R.S. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 5, 11–25 (2004).
Hoeffer, C.A. & Klann, E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 33, 67–75 (2010).
Takei, N. et al. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J. Neurosci. 24, 9760–9769 (2004).
Magariños, A.M. et al. Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus 21, 253–264 (2011).
Chen, Z.-Y. et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science 314, 140–143 (2006).
Bueller, J.A. et al. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol. Psychiatry 59, 812–815 (2006).
Pezawas, L. et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J. Neurosci. 24, 10099–10102 (2004).
Szeszko, P.R. et al. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol. Psychiatry 10, 631–636 (2005).
Kang, H.J. et al. Gene expression profiling in postmortem prefrontal cortex of major depressive disorder. J. Neurosci. 27, 13329–13340 (2007).
Sibille, E., Morris, H.M., Kota, R.S. & Lewis, D.A. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int. J. Neuropsychopharmacol. 14, 721–734 (2011).
Acknowledgements
This work is supported by US National Institutes of Health NIMH R37MH45481 (R.S.D.), NIMH R01MH93897 (R.S.D.), NIMH F32MH98513 (K.T.O.) and NIGMS P30GM103328 (C.A.S.), the State of Connecticut and Yale University. We thank the families consenting to donate brain tissue and be interviewed for the human tissue samples and the Cuyahoga County Medical Examiner's Office for assistance. We thank G. Rajkowska for identification of anatomically comparable regions of dlPFC. We thank Quark Pharmaceuticals for providing the REDD1 plasmid and REDD1-knockout mice. We thank M. Banasr for helpful discussions on behavioral experiments, X.-Y. Li for assistance in breeding and genotyping REDD1-knockout mice, and A. Lepack, W. Andres and Z. LaPalombara for technical assistance. We thank J. Taylor, M. Picciotto and A. Nairn for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
K.T.O. prepared the original draft of the manuscript and was involved in all aspects of the experimental design and research, including execution and analysis of all behavioral, biochemical and molecular experiments, rodent surgeries and dissections and design, construction and preparation of recombinant AAVs. R.-J.L. performed all electrophysiological recordings and neurobiotin spine density analyses. B.V. assisted with optimization and preparation of the recombinant AAVs and with rat surgeries. J.G.M.-A. and R.J.D. assisted in the design and construction of the overexpression construct. V.D. assisted with quantitative PCR execution and analysis. M.I. assisted with rodent behavioral testing and sample preparation. S.D. and C.D. assisted with sample preparation. S.B. and C.R. were responsible for ESP spine density imaging and analysis. D.A.L. and C.A.S. were responsible for human tissue generation and preparation of relevant human subjects' information. G.K.A. was involved in the analysis and interpretation of the electrophysiological and spine density experiments. R.S.D. was involved in all aspects of study design, data analysis, interpretation of results and preparation of the manuscript and figures. All authors discussed the results presented in the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.A.L. currently receives investigator-initiated research support from Bristol-Myers Squibb and Pfizer and from 2012–2014 served as a consultant in the areas of target identification and validation and new compound development to Autifony, Bristol-Myers Squibb, Concert Pharmaceuticals and Sunovion.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 and Supplementary Tables 1–2. (PDF 1373 kb)
Rights and permissions
About this article
Cite this article
Ota, K., Liu, RJ., Voleti, B. et al. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat Med 20, 531–535 (2014). https://doi.org/10.1038/nm.3513
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3513
This article is cited by
-
Neuroimaging in schizophrenia: an overview of findings and their implications for synaptic changes
Neuropsychopharmacology (2023)
-
The stress-responsive protein REDD1 and its pathophysiological functions
Experimental & Molecular Medicine (2023)
-
Repressed Blautia-acetate immunological axis underlies breast cancer progression promoted by chronic stress
Nature Communications (2023)
-
Echinacoside exhibits antidepressant-like effects through AMPAR–Akt/ERK–mTOR pathway stimulation and BDNF expression in mice
Chinese Medicine (2022)
-
Imaging the effect of ketamine on synaptic density (SV2A) in the living brain
Molecular Psychiatry (2022)