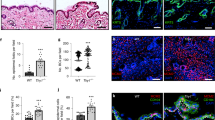
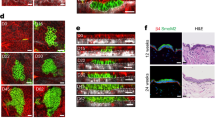
Abstract
Kindlin-1 is an integrin tail binding protein that controls integrin activation. Mutations in the FERMT-1 gene, which encodes for Kindlin-1, lead to Kindler syndrome in man, which is characterized by skin blistering, premature skin aging and skin cancer of unknown etiology. Here we show that loss of Kindlin-1 in mouse keratinocytes recapitulates Kindler syndrome and also produces enlarged and hyperactive stem cell compartments, which lead to hyperthickened epidermis, ectopic hair follicle development and increased skin tumor susceptibility. Mechanistically, Kindlin-1 controls keratinocyte adhesion through β1-class integrins and proliferation and differentiation of cutaneous epithelial stem cells by promoting αvβ6 integrin–mediated transforming growth factor-β (TGF-β) activation and inhibiting Wnt–β-catenin signaling through integrin-independent regulation of Wnt ligand expression. Our findings assign Kindlin-1 the previously unknown and essential task of controlling cutaneous epithelial stem cell homeostasis by balancing TGF-β–mediated growth-inhibitory signals and Wnt–β-catenin–mediated growth-promoting signals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Meves, A., Stremmel, C., Gottschalk, K. & Fässler, R. The Kindlin protein family: new members to the club of focal adhesion proteins. Trends Cell Biol. 19, 504–513 (2009).
Lai-Cheong, J.E. et al. Kindler syndrome: a focal adhesion genodermatosis. Br. J. Dermatol. 160, 233–242 (2009).
Ussar, S. et al. Loss of Kindlin-1 causes skin atrophy and lethal neonatal intestinal epithelial dysfunction. PLoS Genet. 4, e1000289 (2008).
Montanez, E. et al. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 22, 1325–1330 (2008).
Moser, M., Nieswandt, B., Ussar, S., Pozgajova, M. & Fässler, R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat. Med. 14, 325–330 (2008).
Dowling, J.J., Vreede, A.P., Kim, S., Golden, J. & Feldman, E.L. Kindlin-2 is required for myocyte elongation and is essential for myogenesis. BMC Cell Biol. 9, 36 (2008).
Lai-Cheong, J.E., Ussar, S., Arita, K., Hart, I.R. & McGrath, J.A. Colocalization of kindlin-1, kindlin-2, and migfilin at keratinocyte focal adhesion and relevance to the pathophysiology of Kindler syndrome. J. Invest. Dermatol. 128, 2156–2165 (2008).
He, Y., Esser, P., Heinemann, A., Bruckner-Tuderman, L. & Has, C. Kindlin-1 and -2 have overlapping functions in epithelial cells implications for phenotype modification. Am. J. Pathol. 178, 975–982 (2011).
Watt, F.M. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 21, 3919–3926 (2002).
Tumbar, T. et al. Defining the epithelial stem cell niche in skin. Science 303, 359–363 (2004).
Watt, F.M. & Jensen, K.B. Epidermal stem cell diversity and quiescence. EMBO Mol. Med. 1, 260–267 (2009).
Woo, W.M. & Oro, A.E. SnapShot: hair follicle stem cells. Cell 146, 334–334 (2011).
Arwert, E.N., Hoste, E. & Watt, F.M. Epithelial stem cells, wound healing and cancer. Nat. Rev. Cancer 12, 170–180 (2012).
Alonso, L. & Fuchs, E. Stem cells in the skin: waste not, Wnt not. Genes Dev. 17, 1189–1200 (2003).
Watt, F.M. & Fujiwara, H. Cell–extracellular matrix interactions in normal and diseased skin. Cold Spring Harb. Perspect. Biol. 3, a005124 (2011).
Janes, S.M. & Watt, F.M. New roles for integrins in squamous-cell carcinoma. Nat. Rev. Cancer 6, 175–183 (2006).
Evans, R.D. et al. A tumor-associated β1 integrin mutation that abrogates epithelial differentiation control. J. Cell Biol. 160, 589–596 (2003).
Ferreira, M., Fujiwara, H., Morita, K. & Watt, F.M. An activating β1 integrin mutation increases the conversion of benign to malignant skin tumors. Cancer Res. 69, 1334–1342 (2009).
White, D.E. et al. Targeted disruption of β1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell 6, 159–170 (2004).
Yu, Y. et al. Kindlin 2 forms a transcriptional complex with β-catenin and TCF4 to enhance Wnt signalling. EMBO Rep. 13, 750–758 (2012).
Arita, K. et al. Unusual molecular findings in Kindler syndrome. Br. J. Dermatol. 157, 1252–1256 (2007).
Emanuel, P.O., Rudikoff, D. & Phelps, R.G. Aggressive squamous cell carcinoma in Kindler syndrome. Skinmed 5, 305–307 (2006).
Lai-Cheong, J.E. et al. Loss-of-function FERMT1 mutations in kindler syndrome implicate a role for fermitin family homolog-1 in integrin activation. Am. J. Pathol. 175, 1431–1441 (2009).
Has, C. et al. Kindlin-1 is required for RhoGTPase-mediated lamellipodia formation in keratinocytes. Am. J. Pathol. 175, 1442–1452 (2009).
Brakebusch, C. et al. Skin and hair follicle integrity is crucially dependent on β1 integrin expression on keratinocytes. EMBO J. 19, 3990–4003 (2000).
Böttcher, R.T. et al. Sorting nexin 17 prevents lysosomal degradation of β1 integrins by binding to the β1-integrin tail. Nat. Cell Biol. 14, 584–592 (2012).
Braun, K.M. et al. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development 130, 5241–5255 (2003).
Fujiwara, H. et al. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell 144, 577–589 (2011).
Alonso, L. & Fuchs, E. Stem cells of the skin epithelium. Proc. Natl. Acad. Sci. USA 100 (suppl. 1), 11830–11835 (2003).
Jensen, K.B. et al. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell 4, 427–439 (2009).
Munger, J.S. et al. The integrin αvβ6 binds and activates latent TGFβ1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96, 319–328 (1999).
Xie, Y., McElwee, K.J., Owen, G.R., Häkkinen, L. & Larjava, H.S. Integrin β6-deficient mice show enhanced keratinocyte proliferation and retarded hair follicle regression after depilation. J. Invest. Dermatol. 132, 547–555 (2012).
Li, L. & Bhatia, R. Stem cell quiescence. Clin. Cancer Res. 17, 4936–4941 (2011).
Annes, J.P., Chen, Y., Munger, J.S. & Rifkin, D.B. Integrin αVβ6-mediated activation of latent TGF-β requires the latent TGF-β binding protein-1. J. Cell Biol. 165, 723–734 (2004).
Kobielak, K., Stokes, N., de la Cruz, J., Polak, L. & Fuchs, E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc. Natl. Acad. Sci. USA 104, 10063–10068 (2007).
Zhang, J. et al. Bone morphogenetic protein signaling inhibits hair follicle anagen induction by restricting epithelial stem/progenitor cell activation and expansion. Stem Cells 24, 2826–2839 (2006).
Gat, U., DasGupta, R., Degenstein, L. & Fuchs, E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell 95, 605–614 (1998).
Lo Celso, C., Prowse, D.M. & Watt, F.M. Transient activation of β-catenin signaling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development 13, 1787–1799 (2004).
Lowry, W.E. et al. Defining the impact of β-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 19, 1596–1611 (2005).
Silva-Vargas, V. et al. β-catenin and Hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Dev. Cell 9, 121–131 (2005).
Zhou, P., Byrne, C., Jacobs, J. & Fuchs, E. Lymphoid enhancer factor 1 directs hair follicle patterning and epithelial cell fate. Genes Dev. 9, 700–713 (1995).
Estrach, S. et al. Jagged 1 is a β-catenin target gene required for ectopic hair follicle formation in adult epidermis. Development 133, 4427–4438 (2006).
DasGupta, R. & Fuchs, E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126, 4557–4568 (1999).
Bernard, P., Fleming, A., Lacombe, A., Harley, V.R. & Vilain, E. Wnt4 inhibits β-catenin/TCF signalling by redirecting β-catenin to the cell membrane. Biol. Cell 100, 167–177 (2008).
Mikels, A.J. & Nusse, R. Purified Wnt5a protein activates or inhibits β-catenin–TCF signaling depending on receptor context. PLoS Biol. 4, e115 (2006).
Chen, B. et al. Small molecule–mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 5, 100–107 (2009).
Huang, S.M. et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461, 614–620 (2009).
Devgan, V., Mammucari, C., Millar, S.E., Brisken, C. & Dotto, G.P. p21WAF1/Cip1 is a negative transcriptional regulator of Wnt4 expression downstream of Notch1 activation. Genes Dev. 19, 1485–1495 (2005).
Guasch, G. et al. Loss of TGFβ signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell 12, 313–327 (2007).
Beronja, S. et al. RNAi screens in mice identify physiological regulators of oncogenic growth. Nature 501, 185–190 (2013).
Bandyopadhyay, A., Rothschild, G., Kim, S., Calderwood, D.A. & Raghavan, S. Functional differences between kindlin-1 and kindlin-2 in keratinocytes. J. Cell Sci. 125, 2172–2184 (2012).
Ussar, S., Wang, H.V., Linder, S., Fässler, R. & Moser, M. The Kindlins: subcellular localization and expression during murine development. Exp. Cell Res. 312, 3142–3151 (2006).
Oshimori, N. & Fuchs, E. Paracrine TGF-β signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell 10, 63–75 (2012).
Annes, J.P. et al. Making sense of latent TGFβ activation. J. Cell Sci. 116, 217–224 (2003).
Sin, S. et al. Role of the focal adhesion protein kindlin-1 in breast cancer growth and lung metastasis. J. Natl. Cancer Inst. 103, 1323–1337 (2011).
Chaudhury, A. & Howe, P.H. The tale of transforming growth factor-β (TGFβ) signaling: a soigné enigma. IUBMB Life 61, 929–939 (2009).
Fässler, R. & Meyer, M. Consequences of lack of β1 integrin gene expression in mice. Genes Dev. 9, 1896–1908 (1995).
Ramirez, A. et al. A keratin K5Cre transgenic line appropriate for tissue-specific or generalized Cre-mediated recombination. Genesis 39, 52–57 (2004).
Abel, E.L., Angel, J.M., Kiguchi, K. & DiGiovanni, J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat. Protoc. 4, 1350–1362 (2009).
Kasper, M. et al. Wounding enhances epidermal tumorigenesis by recruiting hair follicle keratinocytes. Proc. Natl. Acad. Sci. USA 108, 4099–4104 (2011).
Sundberg, J.P., Sundberg, B.A. & Beamer, W.G. Comparison of chemical carcinogen skin tumor induction efficacy in inbred, mutant, and hybrid strains of mice: morphologic variations of induced tumors and absence of a papillomavirus cocarcinogen. Mol. Carcinog. 20, 19–32 (1997).
Jensen, K.B., Driskell, R.R. & Watt, F.M. Assaying proliferation and differentiation capacity of stem cells using disaggregated adult mouse epidermis. Nat. Protoc. 5, 898–911 (2010).
Lorenz, K. et al. Integrin-linked kinase is required for epidermal and hair follicle morphogenesis. J. Cell Biol. 177, 501–513 (2007).
Kunder, C.A. et al. Mast cell–derived particles deliver peripheral signals to remote lymph nodes. J. Exp. Med. 206, 2455–2467 (2009).
Montanez, E. et al. Analysis of integrin functions in peri-implantation embryos, hematopoietic system, and skin. Methods Enzymol. 426, 239–289 (2007).
Mátés, L. et al. Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat. Genet. 41, 753–761 (2009).
Brown, J.K. et al. Integrin-αvβ6, a putative receptor for foot-and-mouth disease virus, is constitutively expressed in ruminant airways. J. Histochem. Cytochem. 54, 807–816 (2006).
Müller-Röver, S. et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Invest. Dermatol. 117, 3–15 (2001).
Paus, R. et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J. Invest. Dermatol. 113, 523–532 (1999).
Shi, Q. & Boettiger, D. A novel mode for integrin-mediated signaling: tethering is required for phosphorylation of FAK Y397. Mol. Biol. Cell 14, 4306–4315 (2003).
Blümmel, J. et al. Protein repellent properties of covalently attached PEG coatings on nanostructured SiO2-based interfaces. Biomaterials 28, 4739–4747 (2007).
Morales-Avila, E. et al. Multimeric system of 99mTc-labeled gold nanoparticles conjugated to c[RGDfK(C)] for molecular imaging of tumor αvβ3 expression. Bioconjug. Chem. 22, 913–922 (2011).
Acknowledgements
We thank J. Polleux for generating gold nanoarrays, S. Bach for expert technical assistance, C. Mein (Barts and the London Genome Centre) for generating the human microarray data and R. Zent and R. Paus for carefully reading the manuscript. We thank M. Aumailley (University of Cologne), R. Grosschedl (Max Planck Institute (MPI) Immunobiology), S. Violette (Biogen Idec), D. Sheppard (University of California, San Francisco) and M. Wegner (University of Erlangen) for providing antibodies and I. Thesleff (University of Helsinki), R. Kageyama (Kyoto University), A. Kispert (University of Hannover) and J. Behrens (University of Erlangen) for sending essential constructs. This work was funded by the US National Institutes of Health (CA034282) to D.B.R., the Wellcome Trust (PhD studentship to J.E.L.-C.) and the UK National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St. Thomas' National Health Service Foundation Trust and King's College London to J.A.M., the Advanced European Research Council (ERC) Grant (ERC Grant Agreement 322652) and the Max Planck Society to R.F.
Author information
Authors and Affiliations
Contributions
R.F. initiated the project. R.F. and E.R. designed the experiments and wrote the paper. E.R., D.K., M.W., M.J., R.R., S.U., R.T.B. and J.E.L.-C. performed experiments. E.R., M.W., M.J. and R.F. analyzed data. D.B.R. and J.A.M. provided important reagents and/or analytical tools. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 1, 3 and 4. (PDF 23014 kb)
Supplementary Table 2
Microarray data with significant gene expression changes of 2 fold. First sheet shows all genes sorted by the difference score. In the following sheets genes are divided in the indicated categories (Wnt signaling; Inflammation and Wound healing; Proliferation and Cell cycle; Metabolism). N, NHS skin; K, KS skin; AVG, average; DiffScor, difference score. (XLSX 126 kb)
Rights and permissions
About this article
Cite this article
Rognoni, E., Widmaier, M., Jakobson, M. et al. Kindlin-1 controls Wnt and TGF-β availability to regulate cutaneous stem cell proliferation. Nat Med 20, 350–359 (2014). https://doi.org/10.1038/nm.3490
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3490
This article is cited by
-
Advances in cutaneous squamous cell carcinoma
Nature Reviews Cancer (2023)
-
FERMT1 contributes to the migration and invasion of nasopharyngeal carcinoma through epithelial–mesenchymal transition and cell cycle arrest
Cancer Cell International (2022)
-
Identification of FERMT1 and SGCD as key marker in acute aortic dissection from the perspective of predictive, preventive, and personalized medicine
EPMA Journal (2022)
-
FERMT3 mediates cigarette smoke-induced epithelial–mesenchymal transition through Wnt/β-catenin signaling
Respiratory Research (2021)
-
Epidermolysis bullosa
Nature Reviews Disease Primers (2020)